Conversion from chronic to episodic migraine in patients treated with galcanezumab in real life in Italy: the 12-month observational, longitudinal, cohort multicenter GARLIT experience
- PMID: 35763113
- PMCID: PMC9243974
- DOI: 10.1007/s00415-022-11226-4
Conversion from chronic to episodic migraine in patients treated with galcanezumab in real life in Italy: the 12-month observational, longitudinal, cohort multicenter GARLIT experience
Abstract
Objective: To investigate in real-life the conversion from chronic migraine (CM) to episodic migraine (EM), specifically to EM with High-Frequency (HFEM: 8-14 monthly migraine days, MMDs), Medium-Frequency (MFEM, 4-7 MMDs), and Low-Frequency EM (LFEM, 0-3 MMDs), and its persistence during 1 year of treatment with galcanezumab.
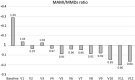
Methods: Consecutive CM patients treated with galcanezumab completing 1 year of observation were enrolled. We collected data on MMDs, pain intensity (Numeric Rating Scale, NRS score), and monthly acute medication intake (MAMI) from baseline (V1) to the 12-month visit (V12).
Results: Of the 155 enrolled patients, 116 (around 75%) reverted to EM at every visit and 81 (52.3%) for the entire 1-year treatment. Patients with older onset age (p = 0.010) and fewer baseline MMDs (p = 0.005) reverted more frequently to EM. At V12, 83 participants (53.5%) presented MFEM or LFEM. Patients reverted to MFEM or LFEM for 7 months (25th 1, 75th 11). The medication overuse discontinuation rate at V12 was 82.8% and occurred for 11 months (25th 8, 75th 12). From baseline to V12, the MAMI decreased by 17 symptomatic drugs (p < 0.000001) while the NRS score reduced by almost 2 points (p < 0.000001). A consistent transition to EM for the entire treatment year was observed in 81 (52.3%) patients.
Discussion: The 1-year GARLIT experience suggests that more than half of CM patients treated with galcanezumab persistently reverted to EM in real life.
Trial registration: ClinicalTrials.gov NCT04803513.
Keywords: Calcitonin gene-related peptide; Chronic migraine; Conversion; Migraine treatment; Monoclonal antibodies; Real world.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany.
Conflict of interest statement
Maria Albanese received honoraria or travel grants from Novartis, Teva, Merck Serono; Claudia Altamura received travel grants and honoraria from Novartis, Eli Lilly, Lusofarmaco, Laborest, Allergan, Almirall; Cinzia Aurilia received travel grants and honoraria from FB-Health, Lusofarmaco, Almirall, Eli-Lilly Novartis and Teva; Piero Barbanti received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Alder, Allergan, Angelini, Bayer, ElectroCore, Eli-Lilly, GSK, Lusofarmaco, MSD, Novartis, Stx-Med, Teva, Visufarma, Zambon; Francesco Bono received honoraria as a speaker or for participating in advisory boards from Teva, Novartis, Ipsen; Sabina Cevoli received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Novartis, Teva, Lilly, Allergan, Ibsa, Amgen and Lundbeck; Vittorio Di Piero received grants and honoraria by Bayer, Biogen, Lilly, TEVA and Novartis; Florindo d’Onofrio received grants and honoraria from Lilly, Teva, Novartis, Neopharmed; Alberto Doretti received grants and honoraria from Novartis, Eli Lilly; Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta and Ecupharma; Valentina Favoni received honoraria as speaker or for participating in advisory boards from Ely-Lilly, Novartis and Teva; Cinzia Finocchi received grants and honoraria from Novartis, Eli Lilly, AIM group; Carlo Lovati received grants from Novartis and Lilly. Florindo d’Onofrio received grants and honoraria from Lilly, Teva, Novartis, Neopharmed; Ilaria Maestrini received honoraria from Eli Lilly. Roberta Messina received honoraria as speaker from Novartis, Eli Lilly, and Teva. Armando Perrotta travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Eli-Lilly, Novartis, and Teva; Angelo Ranieri received speaker honoraria from Teva, Lilly; Fabrizio Vernieri received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Amgen, Angelini, Eli-Lilly, Lundbeck, Novartis, and Teva; Nicoletta Brunelli, Marilena Marcosano and Francesca Schiano Di Cola, Davide Bertuzzo have nothing to disclose.
Figures




References
-
- Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, Lifting the Burden: the Global Campaign against Headache Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi: 10.1186/s10194-020-01208-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

