Maternal antenatal vitamin D supplementation and offspring risk of atopic eczema in the first 4 years of life: evidence from a randomized controlled trial
- PMID: 35763390
- PMCID: PMC9804289
- DOI: 10.1111/bjd.21721
Maternal antenatal vitamin D supplementation and offspring risk of atopic eczema in the first 4 years of life: evidence from a randomized controlled trial
Abstract
Background: Evidence linking prenatal maternal vitamin D supplementation with the offspring's risk of atopic eczema is inconsistent, with most data coming from observational studies.
Objectives: To examine the influence of maternal cholecalciferol supplementation during pregnancy on the risk of atopic eczema in the offspring at ages 12, 24 and 48 months.
Methods: Within the UK Maternal Vitamin D Osteoporosis Study (MAVIDOS) double-blind, randomized placebo-controlled trial, we examined the relationship of maternal vitamin D supplementation during pregnancy with offspring atopic eczema at ages 12, 24 and 48 months. In MAVIDOS, pregnant women were allocated to either cholecalciferol 1000 IU per day or matched placebo, taken from around 14 weeks' gestation until delivery, with the primary outcome of neonatal whole-body bone mineral content. The prevalence of atopic eczema in the offspring was ascertained at ages 12 (n = 635), 24 (n = 610) and 48 (n = 449) months, based on the UK Working Party criteria for the definition of atopic dermatitis. The trial was registered with ISRCTN (82927713) and EudraCT (2007-001716-23).
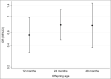
Results: The characteristics of mothers and offspring were similar between the intervention and placebo groups, apart from longer breastfeeding duration in the intervention group. Adjusting for breastfeeding duration, offspring of mothers who received cholecalciferol 1000 IU daily had a lower odds ratio (OR) of atopic eczema at age 12 months [OR 0·55, 95% confidence interval (CI) 0·32-0·97, P = 0·04]; this effect weakened and was not statistically significant at ages 24 months (OR 0·76, 95% CI 0·47-1·23) or 48 months (OR 0·75, 95% CI 0·37-1·52). The statistical interaction of intervention and breastfeeding duration in relation to eczema at age 12 months was not significant (P = 0·41), but stratification showed reduced infantile eczema risk in the intervention group for infants breastfed for ≥ 1 month (OR 0·48, 95% CI 0·24-0·94, P = 0·03) but not in those breastfed for < 1 month (OR 0·80, 95% CI 0·29-2·17, P = 0·66).
Conclusions: Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on the risk of infantile atopic eczema, with the effect potentially being via increased breast milk cholecalciferol levels. The findings support a developmental influence on atopic eczema, and point to a potentially modifiable perinatal influence on atopic eczema. What is already known about this topic? There are currently no antenatal interventions proven to reduce the incidence of infantile atopic eczema in the general population. However, observational studies have led to speculation that antenatal vitamin D supplementation may be beneficial.
© 2022 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
K.M.G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products unrelated to the vitamin D supplement trialled in this study; and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. C.C. reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda, outside the submitted work. N.C.H. reports personal fees, consultancy fees, lecture fees and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, Radius Health, UCB, Consilient Healthcare and Internis Pharma, outside the submitted work. The other authors declare they have no conflicts of interest.
Figures
Comment in
-
Preventing atopic eczema: vitamin D supplementation another piece of the puzzle?Br J Dermatol. 2022 Nov;187(5):630-631. doi: 10.1111/bjd.21806. Epub 2022 Aug 20. Br J Dermatol. 2022. PMID: 35986638 No abstract available.
References
-
- Simpson CR, Anderson WJ, Helms PJ et al. Coincidence of immune‐mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology. A population‐based study using computerized general practice data. Clin Exp Allergy 2002; 32:37–42. - PubMed
-
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998; 139:73–6. - PubMed
-
- Asher MI, Montefort S, Bjorksten B et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006; 368:733–43. - PubMed
-
- Javaid MK, Crozier SR, Harvey NC et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006; 367:36–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 17702/VAC_/Versus Arthritis/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- 21231/VAC_/Versus Arthritis/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/4/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_PC_15015/MRC_/Medical Research Council/United Kingdom
- 201222/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_21002/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical