Metabolome analysis of genus Forsythia related constituents in Forsythia suspensa leaves and fruits using UPLC-ESI-QQQ-MS/MS technique
- PMID: 35763534
- PMCID: PMC9239459
- DOI: 10.1371/journal.pone.0269915
Metabolome analysis of genus Forsythia related constituents in Forsythia suspensa leaves and fruits using UPLC-ESI-QQQ-MS/MS technique
Abstract
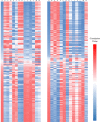
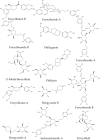
Forsythia suspensa is a traditional Chinese herb. Its numerous metabolites have important roles, as they possessed a wide range of biological activities. This study explored the accumulations of F. suspensa metabolites by performing widely targeted metabolomic analysis. The metabolites were studied at four stages of fruit development. Metabolites in the fruits and leaves of F. suspensa during fruit development included phenolic acids, flavonoids, lipids, lignans and coumarins, amino acids and their derivatives, terpenes, organic acids, nucleotides and their derivatives, alkaloids, quinones, steroids, and tannins. Fourteen Forsythia related metabolites were detected. Their contents varied among the developmental stages. Statistically significant correlations were found between the levels of forsythoside B and 11-methyl-forsythide, and forsythialan B and phillygenin, in both leaves and fruits. According to the correlation analysis between metabolites, Forsythia related metabolites were divided into two classes and five subclasses. In total, 33 compounds presented significant correlations in both fruits and leaves, which indicated the potential relationship in the synthesis of Forsythia related metabolites. Forsythialan B and phillygenin were both negatively correlated with L-valine, while Z-6,7-epoxyligustilid was positively correlated with both compounds. The quality control compounds forsythiaside A and phillyrin were positively and negatively correlated with uracil, respectively. These metabolomics results may facilitate the biosynthesis of Forsythia related metabolites.
Conflict of interest statement
X.C. and Shijiazhuang Yiling Yiling Pharmaceutical Co,Ltd did not develop products and apply patents. X.C. does not hold a dissenting opinion as being a co-author in this study. Each co-author has no potentially competing interests occurred within 5 years of conducting the research under consideration, or preparing the article for publication.This does not alter our adherence to Plos One policies on sharing data and materials.
Figures



References
-
- Guo H, Liu AH, Ye M, Yang M, Guo DA. Characterization of phenolic compounds in the fruits of Forsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007; 21(5): 715–29. doi: 10.1002/rcm.2875 . - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

