Oxytocin receptors are widely distributed in the prairie vole (Microtus ochrogaster) brain: Relation to social behavior, genetic polymorphisms, and the dopamine system
- PMID: 35763609
- PMCID: PMC9474670
- DOI: 10.1002/cne.25382
Oxytocin receptors are widely distributed in the prairie vole (Microtus ochrogaster) brain: Relation to social behavior, genetic polymorphisms, and the dopamine system
Abstract
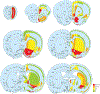
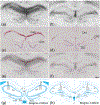
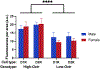
Oxytocin regulates social behavior via direct modulation of neurons, regulation of neural network activity, and interaction with other neurotransmitter systems. The behavioral effects of oxytocin signaling are determined by the species-specific distribution of brain oxytocin receptors. The socially monogamous prairie vole has been a useful model organism for elucidating the role of oxytocin in social behaviors, including pair bonding, response to social loss, and consoling. However, there has been no comprehensive mapping of oxytocin receptor-expressing cells throughout the prairie vole brain. Here, we employed a highly sensitive in situ hybridization, RNAscope, to construct an exhaustive, brain-wide map of oxytocin receptor mRNA-expressing cells. We found that oxytocin receptor mRNA expression was widespread and diffused throughout the brain, with specific areas displaying a particularly robust expression. Comparing receptor binding with mRNA revealed that regions of the hippocampus and substantia nigra contained oxytocin receptor protein but lacked mRNA, indicating that oxytocin receptors can be transported to distal neuronal processes, consistent with presynaptic oxytocin receptor functions. In the nucleus accumbens, a region involved in oxytocin-dependent social bonding, oxytocin receptor mRNA expression was detected in both the D1 and D2 dopamine receptor-expressing subtypes of cells. Furthermore, natural genetic polymorphisms robustly influenced oxytocin receptor expression in both D1 and D2 receptor cell types in the nucleus accumbens. Collectively, our findings further elucidate the extent to which oxytocin signaling is capable of influencing brain-wide neural activity, responses to social stimuli, and social behavior. KEY POINTS: Oxytocin receptor mRNA is diffusely expressed throughout the brain, with strong expression concentrated in certain areas involved in social behavior. Oxytocin receptor mRNA expression and protein localization are misaligned in some areas, indicating that the receptor protein may be transported to distal processes. In the nucleus accumbens, oxytocin receptors are expressed on cells expressing both D1 and D2 dopamine receptor subtypes, and the majority of variation in oxytocin receptor expression between animals is attributable to polymorphisms in the oxytocin receptor gene.
Keywords: Oxtr single nucleotide polymorphisms; autoradiography; dopamine receptor; in situ hybridization; nucleus accumbens; oxytocin receptors mRNA; phenotypic plasticity.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Figures





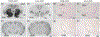

Similar articles
-
Mapping the cellular basis of species differences in oxytocin and dopamine receptor expression in the vole nucleus accumbens.Sci Rep. 2025 Jul 24;15(1):26908. doi: 10.1038/s41598-025-06367-1. Sci Rep. 2025. PMID: 40707636 Free PMC article.
-
Investigation of Oxtr-expressing Neurons Projecting to Nucleus Accumbens using Oxtr-ires-Cre Knock-in prairie Voles (Microtus ochrogaster).Neuroscience. 2020 Nov 10;448:312-324. doi: 10.1016/j.neuroscience.2020.08.023. Neuroscience. 2020. PMID: 33092784 Free PMC article.
-
Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles.Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5981-5. doi: 10.1073/pnas.89.13.5981. Proc Natl Acad Sci U S A. 1992. PMID: 1321430 Free PMC article.
-
Oxytocin, vasopressin and pair bonding: implications for autism.Philos Trans R Soc Lond B Biol Sci. 2006 Dec 29;361(1476):2187-98. doi: 10.1098/rstb.2006.1939. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 17118932 Free PMC article. Review.
-
Oxytocin and Social Relationships: From Attachment to Bond Disruption.Curr Top Behav Neurosci. 2018;35:97-117. doi: 10.1007/7854_2017_10. Curr Top Behav Neurosci. 2018. PMID: 28812266 Free PMC article. Review.
Cited by
-
Oxytocin antagonist does not disrupt rabbit maternal behavior despite binding to brain oxytocin receptors.J Neuroendocrinol. 2023 Jul;35(7):e13236. doi: 10.1111/jne.13236. Epub 2023 Feb 10. J Neuroendocrinol. 2023. PMID: 36762715 Free PMC article.
-
Altered projection-specific synaptic remodeling and its modification by oxytocin in an idiopathic autism marmoset model.Commun Biol. 2024 May 27;7(1):642. doi: 10.1038/s42003-024-06345-9. Commun Biol. 2024. PMID: 38802535 Free PMC article.
-
Natural variation in oxytocin receptor signaling causes widespread changes in brain transcription: a link to the natural killer gene complex.bioRxiv [Preprint]. 2023 Oct 27:2023.10.26.564214. doi: 10.1101/2023.10.26.564214. bioRxiv. 2023. PMID: 37961356 Free PMC article. Preprint.
-
Transcription and DNA methylation signatures of paternal behavior in hippocampal dentate gyrus of prairie voles.Sci Rep. 2023 Jul 7;13(1):11020. doi: 10.1038/s41598-023-37521-2. Sci Rep. 2023. PMID: 37419920 Free PMC article.
-
An AAV-CRISPR/Cas9 strategy for gene editing across divergent rodent species: Targeting neural oxytocin receptors as a proof of concept.Sci Adv. 2023 Jun 2;9(22):eadf4950. doi: 10.1126/sciadv.adf4950. Epub 2023 May 31. Sci Adv. 2023. PMID: 37256960 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

