Risk-Based Cervical Consensus Guidelines: Methods to Determine Management if Less Than 5 Years of Data Are Available
- PMID: 35763610
- PMCID: PMC9232276
- DOI: 10.1097/LGT.0000000000000685
Risk-Based Cervical Consensus Guidelines: Methods to Determine Management if Less Than 5 Years of Data Are Available
Abstract
Objectives: In the 2019 ASCCP Risk-Based Management Consensus Guidelines, clinical management decisions are based on immediate and 5-year cervical intraepithelial neoplasia (CIN) 3+ risk estimates. However, data for technologies other than human papillomavirus testing and cytology may be limited to clinical trials and observational studies of shorter duration than 5 years. To enable decisions about 1- or 3-year intervals, 3-year CIN 3+ risk equivalents to 5-year CIN 3+ risk thresholds were generated.
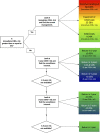
Materials and methods: We examined screening test result scenarios around the 5-year risk thresholds of 0.15% and 0.55% and calculated the average percent increase in CIN 3+ risk from 3 to 5 years. Using this average increase, we obtained estimates of corresponding risk thresholds at 3 years. We then validated whether use of the 3-year risk threshold would have resulted in equivalent management per the 2019 recommendations.
Results: Around the 5-year CIN 3+ risk threshold of 0.55%, the average increase in risk from 3 to 5 years was 0.16%. Therefore, the equivalent threshold for 3-year risk was estimated as 0.39%. We found no difference in recommendations to return in 1 or 3 years using the 3-year or 5-year risk thresholds in 66 of the 67 scenarios (98.5%) in follow-up in 2019 guidelines.
Conclusions: In this methodological addendum, the Enduring Guidelines Committee adopted the use of the 0.39% 3-year CIN 3+ risk threshold as equivalent of the 0.55% 5-year CIN 3+ risk threshold for technologies with fewer than 5 years of follow-up data. This allows evidence-based guidance for surveillance intervals of 1 or 3 years for new technologies with limited longitudinal data.
Copyright © 2022 Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Conflict of interest statement
R.G. is connected with Inovio Pharmaceuticals DSMB and is an ASCCP consultant. W.H. is a Chair and part of Data Safety Monitoring Board of Inovio Pharmaceuticals DSMB; Dr Anna-Barbara Moscicki is part of Merck Global Advisory Board; Dr David Chelmow is a member of the US Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF. The other authors have declared they have no conflicts of interest.
Figures


References
-
- Wentzensen N Arbyn M Berkhof J, et al. . Eurogin 2016 roadmap: how HPV knowledge is changing screening practice. Int J Cancer 2017;140:2192–200. - PubMed

