Treatment Outcomes and Roles of Transplantation and Maintenance Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma: Results From Large Real-World Cohorts
- PMID: 35763708
- PMCID: PMC9870229
- DOI: 10.1200/JCO.21.02698
Treatment Outcomes and Roles of Transplantation and Maintenance Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma: Results From Large Real-World Cohorts
Abstract
Purpose: Commonly used first-line (1L) treatments for mantle cell lymphoma include high-dose cytarabine-based induction followed by autologous stem-cell transplant (ASCT) for younger patients and several chemoimmunotherapy regimens for older patients. Continuous debates exist on the role of ASCT in younger patients and maintenance rituximab (MR) after bendamustine plus rituximab (BR).
Methods: Retrospective data from 4,216 patients with mantle cell lymphoma in the Flatiron Health electronic record-derived deidentified database diagnosed between 2011 and 2021, mostly in US community oncology settings, were evaluated for treatment patterns and outcomes. The efficacy findings with ASCT and MR were validated in an independent cohort of 1,168 patients from 12 academic centers.
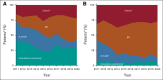
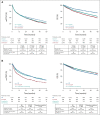
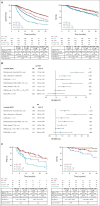
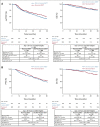
Results: Among 3,614 patients with documented 1L treatment, BR was the most used. Among 1,265 patients age < 65 years, 30.5% received cytarabine-based induction and 23.5% received ASCT. There was no significant association between ASCT and real-world time to next treatment (hazard ratio [HR], 0.84; 95% CI, 0.68 to 1.03; P = .10) or overall survival (HR, 0.86; 95% CI, 0.63 to 1.18; P = .4) among ASCT-eligible patients. Among MR-eligible patients, MR after BR versus BR alone was associated with a longer real-world time to next treatment (HR, 1.96; 95% CI, 1.61 to 2.38; P < .001) and overall survival (HR, 1.51; 95% CI, 1.19 to 1.92; P < .001). The efficacy findings were consistent in the validation cohort.
Conclusion: In this large cohort of patients treated primarily in the US community setting, only one in four young patients received cytarabine or ASCT consolidation, suggesting the need to develop treatments that can be delivered effectively in routine clinical practice. Together with the validation cohort, data support future clinical trials exploring regimens without ASCT consolidation in young patients, whereas MR should be considered for patients after 1L BR and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
Conflict of interest statement
This author is a member of the
No other potential conflicts of interest were reported.
Figures

References
-
- Dreyling M, Campo E, Hermine O, et al. : Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv62-iv71, 2017 - PubMed
-
- Jain P, Wang M: Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 94:710-725, 2019 - PubMed
-
- McKay P, Leach M, Jackson B, et al. : Guideline for the management of mantle cell lymphoma. Br J Haematol 182:46-62, 2018 - PubMed
-
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) B-Cell Lymphomas/Mantle Cell Lymphoma. Version 3.2022, April 25, 2022. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
-
- Rummel M, Knauf W, Goerner M, et al. : Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol 34, 2016. (suppl 15; abstr 7503)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

