The genomic basis of the plant island syndrome in Darwin's giant daisies
- PMID: 35764640
- PMCID: PMC9240058
- DOI: 10.1038/s41467-022-31280-w
The genomic basis of the plant island syndrome in Darwin's giant daisies
Abstract
The repeated, rapid and often pronounced patterns of evolutionary divergence observed in insular plants, or the 'plant island syndrome', include changes in leaf phenotypes, growth, as well as the acquisition of a perennial lifestyle. Here, we sequence and describe the genome of the critically endangered, Galápagos-endemic species Scalesia atractyloides Arnot., obtaining a chromosome-resolved, 3.2-Gbp assembly containing 43,093 candidate gene models. Using a combination of fossil transposable elements, k-mer spectra analyses and orthologue assignment, we identify the two ancestral genomes, and date their divergence and the polyploidization event, concluding that the ancestor of all extant Scalesia species was an allotetraploid. There are a comparable number of genes and transposable elements across the two subgenomes, and while their synteny has been mostly conserved, we find multiple inversions that may have facilitated adaptation. We identify clear signatures of selection across genes associated with vascular development, growth, adaptation to salinity and flowering time, thus finding compelling evidence for a genomic basis of the island syndrome in one of Darwin's giant daisies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
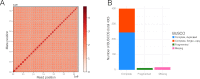
Figures




References
-
- Wallace, A. R. The Malay Archipelago: The Land of the Orang-utan and the Bird of Paradise; a Narrative of Travel, with Studies of Man and Nature (Courier Corporation, 1962).
-
- Mayr, E. Systematics and the Origin of Species from the Viewpoint of a Zoologist (Columbia Uni. Press, 1942).
-
- Emerson BC. Speciation on islands: what are we learning? Biol. J. Linn. Soc. Lond. 2008;95:47–52. doi: 10.1111/j.1095-8312.2008.01120.x. - DOI
-
- Lomolino, M. V., Riddle, B. R., Whittaker, R. J., Brown, J. H. & Lomolino, M. V. Biogeography (Sunderland, Mass: Sinauer Associates, 2017).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

