Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies
- PMID: 35764661
- PMCID: PMC9239993
- DOI: 10.1038/s41541-022-00485-x
Towards the future exploration of mucosal mRNA vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies
Abstract
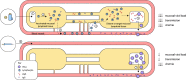
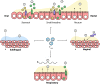
The mRNA vaccine platform has offered the greatest potential in fighting the COVID-19 pandemic owing to rapid development, effectiveness, and scalability to meet the global demand. There are many other mRNA vaccines currently being developed against different emerging viral diseases. As with the current COVID-19 vaccines, these mRNA-based vaccine candidates are being developed for parenteral administration via injections. However, most of the emerging viruses colonize the mucosal surfaces prior to systemic infection making it very crucial to target mucosal immunity. Although parenterally administered vaccines would induce a robust systemic immunity, they often provoke a weak mucosal immunity which may not be effective in preventing mucosal infection. In contrast, mucosal administration potentially offers the dual benefit of inducing potent mucosal and systemic immunity which would be more effective in offering protection against mucosal viral infection. There are however many challenges posed by the mucosal environment which impede successful mucosal vaccination. The development of an effective delivery system remains a major challenge to the successful exploitation of mucosal mRNA vaccination. Nonetheless, a number of delivery vehicles have been experimentally harnessed with different degrees of success in the mucosal delivery of mRNA vaccines. In this review, we provide a comprehensive overview of mRNA vaccines and summarise their application in the fight against emerging viral diseases with particular emphasis on COVID-19 mRNA platforms. Furthermore, we discuss the prospects and challenges of mucosal administration of mRNA-based vaccines, and we explore the existing experimental studies on mucosal mRNA vaccine delivery.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Wang L, Crameri G. Emerging zoonotic viral diseases. Rev. Sci. Tech. 2014;33:569–581. - PubMed
-
- Marston HD, Folkers GK, Morens DM, Fauci AS. Emerging viral diseases: confronting threats with new technologies. Sci. Transl. Med. 2014;6:253ps210–253ps210. - PubMed
-
- Ogbu O, Ajuluchukwu E, Uneke C. Lassa fever in West African sub-region: an overview. J. Vector Borne Dis. 2007;44:1. - PubMed
-
- Hartman AL, Towner JS, Nichol ST. Ebola and marburg hemorrhagic fever. Clin. Lab. Med. 2010;30:161–177. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

