Hypoxia Inducible Factor-1α binds and activates γ-secretase for Aβ production under hypoxia and cerebral hypoperfusion
- PMID: 35764706
- PMCID: PMC9722522
- DOI: 10.1038/s41380-022-01676-7
Hypoxia Inducible Factor-1α binds and activates γ-secretase for Aβ production under hypoxia and cerebral hypoperfusion
Abstract
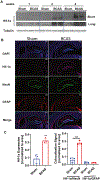
Hypoxic-ischemic injury has been linked with increased risk for developing Alzheimer's disease (AD). The underlying mechanism of this association is poorly understood. Here, we report distinct roles for hypoxia-inducible factor-1α (Hif-1α) in the regulation of BACE1 and γ-secretase activity, two proteases involved in the production of amyloid-beta (Aβ). We have demonstrated that Hif-1α upregulates both BACE1 and γ-secretase activity for Aβ production in brain hypoxia-induced either by cerebral hypoperfusion or breathing 10% O2. Hif-1α binds to γ-secretase, which elevates the amount of active γ-secretase complex without affecting the level of individual subunits in hypoxic-ischemic mouse brains. Additionally, the expression of full length Hif-1α increases BACE1 and γ-secretase activity in primary neuronal culture, whereas a transcriptionally incompetent Hif-1α variant only activates γ-secretase. These findings indicate that Hif-1α transcriptionally upregulates BACE1 and nontranscriptionally activates γ-secretase for Aβ production in hypoxic-ischemic conditions. Consequently, Hif-1α-mediated Aβ production may be an adaptive response to hypoxic-ischemic injury, subsequently leading to increased risk for AD. Preventing the interaction of Hif-1α with γ-secretase may therefore be a promising therapeutic strategy for AD treatment.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
CONFLICT OF INTEREST
LYM is a co-inventor of intellectual property (assay for gamma secretase activity and screening method for gamma secretase inhibitors) owned by MSKCC and licensed to Jiangsu Continental Medical Development. CI serves on the Scientific Advisory Board of Broadview Ventures.
Figures




References
-
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

