Pandemic-proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP)
- PMID: 35764796
- PMCID: PMC9240050
- DOI: 10.1038/s41746-022-00622-9
Pandemic-proof recruitment and engagement in a fully decentralized trial in atrial fibrillation patients (DeTAP)
Abstract
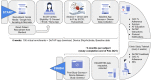
The Coronavirus Disease 2019 (COVID-19) pandemic curtailed clinical trial activity. Decentralized clinical trials (DCTs) can expand trial access and reduce exposure risk but their feasibility remains uncertain. We evaluated DCT feasibility for atrial fibrillation (AF) patients on oral anticoagulation (OAC). DeTAP (Decentralized Trial in Afib Patients, NCT04471623) was a 6-month, single-arm, 100% virtual study of 100 AF patients on OAC aged >55 years, recruited traditionally and through social media. Participants enrolled and participated virtually using a mobile application and remote blood pressure (BP) and six-lead electrocardiogram (ECG) sensors. Four engagement-based primary endpoints included changes in pre- versus end-of-study OAC adherence (OACA), and % completion of televisits, surveys, and ECG and BP measurements. Secondary endpoints included survey-based nuisance bleeding and patient feedback. 100 subjects (mean age 70 years, 44% women, 90% White) were recruited in 28 days (traditional: 6 pts; social media: 94 pts in 12 days with >300 waitlisted). Study engagement was high: 91% televisits, 85% surveys, and 99% ECG and 99% BP measurement completion. OACA was unchanged at 6 months (baseline: 97 ± 9%, 6 months: 96 ± 15%, p = 0.39). In patients with low baseline OACA (<90%), there was significant 6-month improvement (85 ± 16% to 96 ± 6%, p < 0.01). 86% of respondents (69/80) expressed willingness to continue in a longer trial. The DeTAP study demonstrated rapid recruitment, high engagement, and physiologic reporting via the integration of digital technologies and dedicated study coordination. These findings may inform DCT designs for future cardiovascular trials.
© 2022. The Author(s).
Conflict of interest statement
A.S. reports research support from the American Heart Association. U.G., T.V., H.M., and J.L. are employees of Bayer A.G. M.J.T.S. is an employee of Huma Therapeutics Ltd. K.D.L. is an employee of Yuzu Labs PBC. K.W.M.’s financial disclosures can be viewed at
Figures

References
LinkOut - more resources
Full Text Sources
Medical

