Highlighting the Undetectable - Fluorescence Molecular Imaging in Gastrointestinal Endoscopy
- PMID: 35764908
- PMCID: PMC9971088
- DOI: 10.1007/s11307-022-01741-1
Highlighting the Undetectable - Fluorescence Molecular Imaging in Gastrointestinal Endoscopy
Abstract
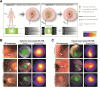
Flexible high-definition white-light endoscopy is the current gold standard in screening for cancer and its precursor lesions in the gastrointestinal tract. However, miss rates are high, especially in populations at high risk for developing gastrointestinal cancer (e.g., inflammatory bowel disease, Lynch syndrome, or Barrett's esophagus) where lesions tend to be flat and subtle. Fluorescence molecular endoscopy (FME) enables intraluminal visualization of (pre)malignant lesions based on specific biomolecular features rather than morphology by using fluorescently labeled molecular probes that bind to specific molecular targets. This strategy has the potential to serve as a valuable tool for the clinician to improve endoscopic lesion detection and real-time clinical decision-making. This narrative review presents an overview of recent advances in FME, focusing on probe development, techniques, and clinical evidence. Future perspectives will also be addressed, such as the use of FME in patient stratification for targeted therapies and potential alliances with artificial intelligence. KEY MESSAGES: • Fluorescence molecular endoscopy is a relatively new technology that enables safe and real-time endoscopic lesion visualization based on specific molecular features rather than on morphology, thereby adding a layer of information to endoscopy, like in PET-CT imaging. • Recently the transition from preclinical to clinical studies has been made, with promising results regarding enhancing detection of flat and subtle lesions in the colon and esophagus. However, clinical evidence needs to be strengthened by larger patient studies with stratified study designs. • In the future fluorescence molecular endoscopy could serve as a valuable tool in clinical workflows to improve detection in high-risk populations like patients with Barrett's esophagus, Lynch syndrome, and inflammatory bowel syndrome, where flat and subtle lesions tend to be malignant up to five times more often. • Fluorescence molecular endoscopy has the potential to assess therapy responsiveness in vivo for targeted therapies, thereby playing a role in personalizing medicine. • To further reduce high miss rates due to human and technical factors, joint application of artificial intelligence and fluorescence molecular endoscopy are likely to generate added value.
Keywords: Artificial intelligence; Cancer; Early detection; Fluorescence; Fluorescence molecular endoscopy; Gastrointestinal endoscopy; Inflammation; Molecular imaging; Near-infrared fluorescence; Optical imaging; Personalized medicine; Targeted biopsy.
© 2022. The Author(s).
Conflict of interest statement
ICMJE forms enclosed. S. R. has a patent for a drug fragment to image precancerous and cancerous lesions (WO2019173483A1). The molecule is not mentioned in this review.
Figures


References
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A et al (2021) Cancer statistics for the year 2020: an overview. Int J Cancer. 10.1002/ijc.33588 - PubMed
-
- Săftoiu A, Hassan C, Areia M, Bhutani MS, Bisschops R, Bories E, et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020;52(4):293–304. doi: 10.1055/a-1104-5245. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

