FGF1 alleviates LPS-induced acute lung injury via suppression of inflammation and oxidative stress
- PMID: 35764933
- PMCID: PMC9238076
- DOI: 10.1186/s10020-022-00502-8
FGF1 alleviates LPS-induced acute lung injury via suppression of inflammation and oxidative stress
Abstract
Background: Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), are devastating clinical disorders with high mortality, and for which more effective therapies are urgently needed. FGF1, the prototype member of the FGF family, is shown to exert protective effects against injurious stimuli in multiple disease models. Here we aimed to evaluate whether FGF1 pretreatment is protective against LPS-induced ALI and elucidate the potential underlying mechanisms.
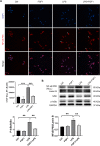
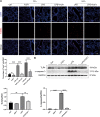
Methods: For drug-treated groups, C57B/6 mice received a single i.p. injection of FGF1 (1 mg/kg) 1 h before the LPS challenge or not. To induce the ALI model, the mice were treated by intratracheal instillation of LPS (5 mg/kg). Then, histopathological changes in lung tissues were assessed by hematoxylin and eosin staining and transmission electron microscopy. ELISA and qPCR assays were used to detect pro-inflammatory cytokine levels in BALF and lung tissues, respectively. The total number of inflammatory cells (neutrophils and macrophages) in BALF were counted using the Wright-Giemsa method. The expressions of reactive oxygen species (ROS) and malondialdehyde (MDA) were measured using their respective kits. Western blot and immunostaining were used to evaluate the expressions of antioxidants (Nrf-2, HO-1, SOD2, GPX4, and Catalase), as well as the inflammatory and/or apoptosis-related factors (TLR4, NF-κB, and Cleaved- caspase 3).
Results: FGF1 pretreatment significantly ameliorated the LPS-induced histopathological changes, reduced lung wet/dry ratios, ROS and MDA levels, total BALF protein, inflammatory cell infiltration, proinflammatory cytokine levels, and significantly increased the expression of antioxidant proteins (Nrf-2, HO-1, Catalase, and SOD2). In addition, FGF1 pretreatment significantly reduced the expression of TLR4 and cleaved- caspase 3, inhibited NF-κB activation, and reduced LPS-induced cell apoptosis.
Conclusions: Altogether, our results suggest that FGF1 pretreatment is protective against LPS-induced ALI through mediating anti-inflammatory and antioxidant effects, which may be attributed to the downregulation of TLR4 expression and inhibition of NF-κB activation, as well as promotion of antioxidant defenses. Therefore, FGF1 administration may prove beneficial in preventative strategies for ALI/ARDS.
Keywords: Acute lung injury; FGF1; Inflammation; Lipopolysaccharide; NF-κB; Nrf2; Oxidative stress; TLR4.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abraham E, Nick JA, Azam T, Kim SH, Mira JP, Svetkauskaite D, He Q, Zamora M, Murphy J, Park JS, et al. Peripheral blood neutrophil activation patterns are associated with pulmonary inflammatory responses to lipopolysaccharide in humans. J Immunol. 2006;176:7753–60. doi: 10.4049/jimmunol.176.12.7753. - DOI - PubMed
-
- Bhattacharya S. Reactive oxygen species and cellular defense system. In Free radicals in human health and disease. 2015;17–29.
-
- Bloomer RJ. Chapter 1 Effect of exercise on oxidative stress biomarkers. 2008;1–50. - PubMed
-
- Ding I, Liu W, Sun J, Paoni SF, Hernady E, Fenton BM, Okunieff P. FGF1 and VEGF mediated angiogenesis in KHT tumor-bearing mice. In: Oxygen transport to tissue XXIV (Springer), 2003; pp. 603–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

