Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice
- PMID: 35764988
- PMCID: PMC9237999
- DOI: 10.1186/s12974-022-02523-w
Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice
Abstract
Background: Gut microbiota has been found involved in neuronal functions and neurological disorders. Whether and how gut microbiota impacts chronic somatic pain disorders remain elusive.
Methods: Neuropathic pain was produced by different forms of injury or diseases, the chronic constriction injury (CCI) of the sciatic nerves, oxaliplatin (OXA) chemotherapy, and streptozocin (STZ)-induced diabetes in mice. Continuous feeding of antibiotics (ABX) cocktail was used to cause major depletion of the gut microbiota. Fecal microbiota, biochemical changes in the spinal cord and dorsal root ganglion (DRG), and the behaviorally expressed painful syndromes were assessed.
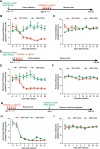
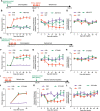
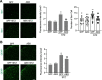
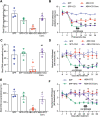
Results: Under condition of gut microbiota depletion, CCI, OXA, or STZ treatment-induced thermal hyperalgesia or mechanical allodynia were prevented or completely suppressed. Gut microbiota depletion also prevented CCI or STZ treatment-induced glial cell activation in the spinal cord and inhibited cytokine production in DRG in OXA model. Interestingly, STZ treatment failed to induce the diabetic high blood glucose and painful hypersensitivity in animals with the gut microbiota depletion. ABX feeding starting simultaneously with CCI, OXA, or STZ treatment resulted in instant analgesia in all the animals. ABX feeding starting after establishment of the neuropathic pain in CCI- and STZ-, but not OXA-treated animals produced significant alleviation of the thermal hyeralgesia or mechanical allodynia. Transplantation of fecal bacteria from SPF mice to ABX-treated mice partially restored the gut microbiota and fully rescued the behaviorally expressed neuropathic pain, of which, Akkermansia, Bacteroides, and Desulfovibrionaceae phylus may play a key role.
Conclusion: This study demonstrates distinct roles of gut microbiota in the pathogenesis of chronic painful conditions with nerve injury, chemotherapy and diabetic neuropathy and supports the clinical significance of fecal bacteria transplantation.
Keywords: Chemotherapy; Diabetes; Gut microbiota; Neuropathic pain.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures





References
MeSH terms
Substances
Grants and funding
- KQTD20200820113040070/Science, Technology and Innovation Commission of Shenzhen Municipality
- JCYJ20200109141433384/The Foundation of Shenzhen Science and Technology Innovation Committee
- 7191001/Natural Science Foundation of Beijing Municipality
- NFSC81971062/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

