Emerging role of exosomes in cancer progression and tumor microenvironment remodeling
- PMID: 35765040
- PMCID: PMC9238168
- DOI: 10.1186/s13045-022-01305-4
Emerging role of exosomes in cancer progression and tumor microenvironment remodeling
Abstract
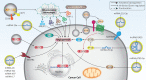
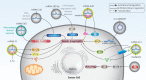
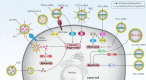
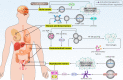
Cancer is one of the leading causes of death worldwide, and the factors responsible for its progression need to be elucidated. Exosomes are structures with an average size of 100 nm that can transport proteins, lipids, and nucleic acids. This review focuses on the role of exosomes in cancer progression and therapy. We discuss how exosomes are able to modulate components of the tumor microenvironment and influence proliferation and migration rates of cancer cells. We also highlight that, depending on their cargo, exosomes can suppress or promote tumor cell progression and can enhance or reduce cancer cell response to radio- and chemo-therapies. In addition, we describe how exosomes can trigger chronic inflammation and lead to immune evasion and tumor progression by focusing on their ability to transfer non-coding RNAs between cells and modulate other molecular signaling pathways such as PTEN and PI3K/Akt in cancer. Subsequently, we discuss the use of exosomes as carriers of anti-tumor agents and genetic tools to control cancer progression. We then discuss the role of tumor-derived exosomes in carcinogenesis. Finally, we devote a section to the study of exosomes as diagnostic and prognostic tools in clinical courses that is important for the treatment of cancer patients. This review provides a comprehensive understanding of the role of exosomes in cancer therapy, focusing on their therapeutic value in cancer progression and remodeling of the tumor microenvironment.
Keywords: Biomarker; Cancer; Exosome; Immunotherapy; Non-coding RNA; Prognosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Cai W, Xiong Chen Z, Rane G, Satendra Singh S, Choo Z, Wang C, et al. Wanted DEAD/H or alive: helicases winding up in cancers. J Natl Cancer Inst. 2017;109(6):djw278. - PubMed
-
- Manu KA, Shanmugam MK, Ramachandran L, Li F, Siveen KS, Chinnathambi A, et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015;363(1):28–36. - PubMed
-
- Wang C, Kar S, Lai X, Cai W, Arfuso F, Sethi G, et al. Triple negative breast cancer in Asia: an insider's view. Cancer Treat Rev. 2018;62:29–38. - PubMed
-
- Li F, Shanmugam MK, Chen L, Chatterjee S, Basha J, Kumar AP, et al. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev Res (Phila) 2013;6(8):843–854. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

