Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation
- PMID: 35765102
- PMCID: PMC9238175
- DOI: 10.1186/s13054-022-04053-6
Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation
Abstract
Background: Severe COVID-19 induced acute respiratory distress syndrome (ARDS) often requires extracorporeal membrane oxygenation (ECMO). Recent German health insurance data revealed low ICU survival rates. Patient characteristics and experience of the ECMO center may determine intensive care unit (ICU) survival. The current study aimed to identify factors affecting ICU survival of COVID-19 ECMO patients.
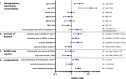
Methods: 673 COVID-19 ARDS ECMO patients treated in 26 centers between January 1st 2020 and March 22nd 2021 were included. Data on clinical characteristics, adjunct therapies, complications, and outcome were documented. Block wise logistic regression analysis was applied to identify variables associated with ICU-survival.
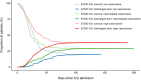
Results: Most patients were between 50 and 70 years of age. PaO2/FiO2 ratio prior to ECMO was 72 mmHg (IQR: 58-99). ICU survival was 31.4%. Survival was significantly lower during the 2nd wave of the COVID-19 pandemic. A subgroup of 284 (42%) patients fulfilling modified EOLIA criteria had a higher survival (38%) (p = 0.0014, OR 0.64 (CI 0.41-0.99)). Survival differed between low, intermediate, and high-volume centers with 20%, 30%, and 38%, respectively (p = 0.0024). Treatment in high volume centers resulted in an odds ratio of 0.55 (CI 0.28-1.02) compared to low volume centers. Additional factors associated with survival were younger age, shorter time between intubation and ECMO initiation, BMI > 35 (compared to < 25), absence of renal replacement therapy or major bleeding/thromboembolic events.
Conclusions: Structural and patient-related factors, including age, comorbidities and ECMO case volume, determined the survival of COVID-19 ECMO. These factors combined with a more liberal ECMO indication during the 2nd wave may explain the reasonably overall low survival rate. Careful selection of patients and treatment in high volume ECMO centers was associated with higher odds of ICU survival.
Trial registration: Registered in the German Clinical Trials Register (study ID: DRKS00022964, retrospectively registered, September 7th 2020, https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00022964 .
Keywords: Acute respiratory distress syndrome (ARDS); COVID-19; Case-volume relationship; Extracorporeal life support; Intensive care.
© 2022. The Author(s).
Conflict of interest statement
Christian Karagiannidis reports support for the present manuscript by Xenios (advisory board member), Getinge (personal fees); personal fees by Bayer, Xenios, Pfizer, Getinge; President of the German Society of internal Intensive Care Medicine; Lead of the German ICU Registry. Stefan Kluge reports consulting fees by Baxter, Fresenius Medical Care; payment by Baxter, Fresenius Medical Care. Karsten Schmidt reports research grants from Stiftung Universitätsmedizin Essen (Microcirculation/Sepsis/Covid-19), Heidelberger Stiftung Chirurgie (Microcirculation/Sepsis); payment for lectures (Buthylcholinesterase and Inflammation) by Dr. Franz Köhler Chemie GmbH. Onnen Moerer reports research grants from CSL Behring: Unrestricted Grant from CS Behring (research related to extracorporeal membrane oxygenation); Member of the national CEOsys network Germany (Covid ecosystem), which is funded by the Federal Ministry of Education and Research (BMBF). [FKZ 01KX2021]; Participation as speaker in workshops on haemodynamic monitoring at the European Medical School in Oldenburg, Germany; Expert testimonies for legal proceedings related to Critical Care or Anesthesiology cases on malpractice accusation by district courts and conciliation committees. Matthias Kochanek reports payment for lectures for Astellas, MSD, Gilead, Pfizer; stock or stock options by Biontech, Moderna; member of DGIIN, DGIM, DIVI. Tobias Lahmer reports payment for lectures and presentations by Gilead, MSD, Pfizer, ADVITOS GmbH. Peter Heuschmann reports research grants from German Ministry of Research and Education, European Union, Charité – Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, German Research Foundation, Bavarian State (ministry for science and the arts), German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside the submitted work. Peter Kranke reports research grants for CEOsys projects in the Network University Medicine (NUM) (institutional grants); chairman of the ESAIC guideline committee. Thorsten Brenner reports research grants from Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Innovationsfonds/Gemeinsamer Bundesauschuss (G-BA), Stiftung Universitäts-medizin Essen; payments by CSL Behring GmbH, Schöchl medical education GmbH, Boehringer Ingelheim Pharma GmbH, Biotest AG, Baxter Deutschland GmbH, Astellas Pharma GmbH, B. Braun Melsungen AG, MSD Sharp & Dohme GmbH, Lücke Kongresse GmbH, Akademie für Infektionsmedizin e.V.; 2 patents on biomarkers in sepsis/septic shock with BRAHMS GmbH. Frank Herbstreit reports speaker honoria by Biotest; paid expert witness at several courts; International Anesthesia Research Society: Support for Attendance of Annual Meeting; Senior Editor: Anesthesia & Analgesia. All other authors declare no conflict of interest.
Figures

References
-
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. - DOI - PMC - PubMed
-
- Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518–526. doi: 10.1016/S2213-2600(20)30121-1. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

