Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial
- PMID: 35765103
- PMCID: PMC9241239
- DOI: 10.1186/s13287-022-02920-1
Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial
Abstract
Background: Acute respiratory distress syndrome (ARDS) is the devastating complication of the new COVID-19 pandemic, directly correlated with releasing large amounts of inflammatory cytokines. Due to their immunoregulatory features, mesenchymal stromal cells (MSCs) provide a promising approach against this disease. In this regard, this study was designed as a single-center, open-label, phase 1 clinical trial with a control group to examine the safety and explore the possible potency of three injections of umbilical cord-derived MSCs (UC-MSCs) in mild-moderate COVID-19-induced ARDS patients.
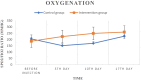
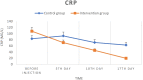
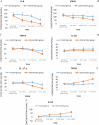
Methods: Twenty confirmed COVID-19 patients with mild-to-moderate ARDS degree entered the study and were divided into two groups: control group (standard care) and intervention group (standard care + UC-MSCs). The patients received three intravenous infusions of UC-MSCs (1 × [Formula: see text] cells/kg BW per injection) every other day. Respiratory markers, CRP levels and specific serum cytokines were assessed four times (days of 0, 5, 10 and 17) during the 17-day follow-up period.
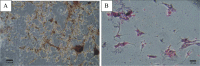
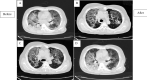
Results: During the study, there were no serious adverse effects after cell transplantations. Besides, significant improvement in SPO2/FIO2 ratio and serum CRP levels was observed. On the other hand, a significant decrease (P < 0.05) in serum cytokine levels of IL-6, IFN-g, TNF-α, IL-17 A and a significant increase in serum cytokine levels of TGF-B, IL-1B and IL-10 were observed. Also, no significant changes were observed in CT scan images of patients during the study period.
Conclusion: Our obtained results demonstrated that multiple intravenous transplantations of allogenic UC-MSCs in non-severe COVID-19-induced ARDS patients are a safe procedure. In addition, this intervention is a hopeful approach to decline cytokine storm and recover respiratory functions. Indeed, more clinical trials with larger sample sizes are required to confirm these results. Trial registration This clinical trial was registered with the Iranian Registry of Clinical Trials (ID: IRCT20160809029275N1 at 2020.05.30).
Keywords: Acute respiratory distress syndrome; Clinical trial; Mesenchymal stromal cells; Safety.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series.Stem Cell Res Ther. 2021 Jan 29;12(1):91. doi: 10.1186/s13287-021-02165-4. Stem Cell Res Ther. 2021. PMID: 33514427 Free PMC article. Clinical Trial.
-
Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment.Stem Cell Res Ther. 2022 Jul 28;13(1):365. doi: 10.1186/s13287-022-02953-6. Stem Cell Res Ther. 2022. PMID: 35902979 Free PMC article. Clinical Trial.
-
Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial.Crit Care. 2022 Feb 21;26(1):48. doi: 10.1186/s13054-022-03930-4. Crit Care. 2022. PMID: 35189925 Free PMC article. Clinical Trial.
-
Mesenchymal stromal cells as treatment for acute respiratory distress syndrome. Case Reports following hematopoietic cell transplantation and a review.Front Immunol. 2022 Nov 8;13:963445. doi: 10.3389/fimmu.2022.963445. eCollection 2022. Front Immunol. 2022. PMID: 36426365 Free PMC article. Review.
-
Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential.Expert Rev Respir Med. 2021 Mar;15(3):301-324. doi: 10.1080/17476348.2021.1848555. Epub 2020 Nov 26. Expert Rev Respir Med. 2021. PMID: 33172313 Review.
Cited by
-
The Art of Stem Cell-Based Therapy.Adv Exp Med Biol. 2023;1420:1-12. doi: 10.1007/978-3-031-30040-0_1. Adv Exp Med Biol. 2023. PMID: 37258780
-
The ARDS microenvironment enhances MSC-induced repair via VEGF in experimental acute lung inflammation.Mol Ther. 2024 Oct 2;32(10):3422-3432. doi: 10.1016/j.ymthe.2024.08.003. Epub 2024 Aug 5. Mol Ther. 2024. PMID: 39108095 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles reduce inflammatory responses to SARS-CoV-2 and Influenza viral proteins via miR-146a/NF-κB pathway.Sci Rep. 2024 Nov 4;14(1):26649. doi: 10.1038/s41598-024-77258-0. Sci Rep. 2024. PMID: 39496662 Free PMC article.
-
Mesenchymal Stem Cell-Based Therapies in the Post-Acute Neurological COVID Syndrome: Current Landscape and Opportunities.Biomolecules. 2023 Dec 20;14(1):8. doi: 10.3390/biom14010008. Biomolecules. 2023. PMID: 38275749 Free PMC article. Review.
-
Evaluating the therapeutic potential of different sources of mesenchymal stem cells in acute respiratory distress syndrome.Stem Cell Res Ther. 2024 Oct 29;15(1):385. doi: 10.1186/s13287-024-03977-w. Stem Cell Res Ther. 2024. PMID: 39468662 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

