Improving Hepatitis B Vaccination Rates among At-risk Children and Adolescents with Inflammatory Bowel Disease
- PMID: 35765569
- PMCID: PMC9225488
- DOI: 10.1097/pq9.0000000000000570
Improving Hepatitis B Vaccination Rates among At-risk Children and Adolescents with Inflammatory Bowel Disease
Erratum in
-
Erratum: Improving Hepatitis B Vaccination Rates among At-risk Children and Adolescents with Inflammatory Bowel Disease: Erratum.Pediatr Qual Saf. 2022 Aug 1;7(4):e584. doi: 10.1097/pq9.0000000000000584. eCollection 2022 Jul-Aug. Pediatr Qual Saf. 2022. PMID: 35928025 Free PMC article.
Abstract
Patients with inflammatory bowel disease (IBD) receiving tumor necrosis factor alpha inhibitors (TNFai) may be at higher risk for hepatitis B virus (HBV) infection. We conducted a quality improvement (QI) initiative to improve HBV vaccination rates in seronegative children with IBD.
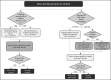
Methods: This QI initiative implemented an HBV vaccination strategy from September 2018 to March 2020 in patients with newly diagnosed IBD with hepatitis B surface antibody (HBsAb) <10 mIU/mL. The project aimed to (1) increase HBV vaccination rates in seronegative patients and (2) document immunogenicity after completing a three-dose vaccine series. Outcome measures included the percentage of seronegative patients who received HBV vaccines (dose 1 and three-dose series). Interventions included applying a standardized vaccination protocol, and creating a vaccine workflow in two clinical areas, previsit planning and stakeholder engagement.
Results: One hundred seventy-four children and adolescents with IBD were evaluated during the study period, and 132 (76%) were HBsAb negative. After plan-do-study-act (PDSA) 1, the proportion of eligible patients who received HBV vaccine dose 1 increased from a baseline of 7% to 100% and was sustained for over 12 months. During PDSA 2, the proportion of patients completing the three-dose vaccine series improved from a baseline of 0% to 82% (n = 100); among 93 children in this subgroup who had repeat serology performed, 86 (92%) demonstrated serologic evidence of HBV protection.
Conclusions: A multidisciplinary approach applying QI methodology allowed for improved and sustained HBV vaccination rates in at-risk seronegative children and adolescents with IBD. A three-dose HBV vaccine series proved immunogenic in 92% of eligible patients.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Figures




References
-
- Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. - PubMed
-
- Ardura MI, Toussi SS, Siegel JD, et al. NASPGHAN clinical report: surveillance, diagnosis, and prevention of infectious diseases in pediatric patients with inflammatory bowel disease receiving tumor necrosis factor-α inhibitors. J Pediatr Gastroenterol Nutr. 2016;63:130–155. - PubMed
-
- Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–244.e3. - PubMed
-
- Reddy KR, Beavers KL, Hammond SP, et al.; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219; quiz e16. - PubMed
-
- Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–S165. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
