Safety and Adverse Events Related to COVID-19 mRNA Vaccines; a Systematic Review
- PMID: 35765616
- PMCID: PMC9206826
- DOI: 10.22037/aaem.v10i1.1597
Safety and Adverse Events Related to COVID-19 mRNA Vaccines; a Systematic Review
Abstract
Introduction: Knowledge of vaccine-related adverse events is crucial as they are among the most important factors that cause hesitation in receiving vaccines. Therefore, we aimed to systematically review the adverse events related to the mRNA vaccines reported in the literature..
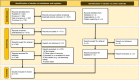
Method: A systematic literature search was carried out in the databases of Scopus, PubMed, Cochrane, and Web of Science. We selected original studies that explored the side effects of mRNA COVID-19 vaccines using a two-phase (title/abstract and full-text) screening process..
Results: Cardiac complications were the most commonly reported severe adverse events. It appeared that systemic adverse reactions are more common after the second dose of vaccines. The number of adverse effects reported after the Pfizer vaccine was higher than other vaccines, mostly due to its earlier approval and more widespread use throughout the world. Cardiac adverse events had a higher prevalence but no significant association has been found between COVID-19 mRNA vaccines and cardiac adverse events except for myopericarditis. .
Conclusion: Vaccines play a crucial role in controlling the COVID-19 pandemic and decreasing mortalities and the results of the present review acknowledge the fact that the benefits outweigh the adverse events of these vaccines..
Keywords: 2019-nCoV Vaccine mRNA-1273; Adverse effects; COVID-19 vaccines; BNT162 Vaccine; mRNA vaccines.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Figures
Similar articles
-
Safety and Adverse Effects Related to COVID-19 Viral Vector Vaccines: A Systematic Review.Tanaffos. 2024 Feb;23(2):102-114. Tanaffos. 2024. PMID: 39959793 Free PMC article. Review.
-
Safety and Adverse Events Related to Inactivated COVID-19 Vaccines and Novavax;a Systematic Review.Arch Acad Emerg Med. 2022 Jul 7;10(1):e54. doi: 10.22037/aaem.v10i1.1585. eCollection 2022. Arch Acad Emerg Med. 2022. PMID: 36033990 Free PMC article. Review.
-
Exploring the adverse events of Oxford-AstraZeneca, Pfizer-BioNTech, Moderna, and Johnson and Johnson COVID-19 vaccination on Guillain-Barré Syndrome.Sci Rep. 2024 Aug 13;14(1):18767. doi: 10.1038/s41598-024-66999-7. Sci Rep. 2024. PMID: 39138276 Free PMC article.
-
COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis.Front Immunol. 2021 Nov 29;12:729251. doi: 10.3389/fimmu.2021.729251. eCollection 2021. Front Immunol. 2021. PMID: 34912330 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
Cited by
-
Safety and efficiency of COVID-19 vaccine in North Africa.Hum Vaccin Immunother. 2024 Dec 31;20(1):2306703. doi: 10.1080/21645515.2024.2306703. Epub 2024 Feb 2. Hum Vaccin Immunother. 2024. PMID: 38304972 Free PMC article.
-
Analysis of articles in the Journal of Archives of Academic Emergency Medicine in 2022.Arch Acad Emerg Med. 2023 Jan 1;11(1):e3. doi: 10.22037/aaem.v11i1.1882. eCollection 2023. Arch Acad Emerg Med. 2023. PMID: 36620732 Free PMC article. No abstract available.
-
Safety and Adverse Effects Related to COVID-19 Viral Vector Vaccines: A Systematic Review.Tanaffos. 2024 Feb;23(2):102-114. Tanaffos. 2024. PMID: 39959793 Free PMC article. Review.
-
Pathophysiology of oral lesions subsequent to SARS-CoV-2 vaccination: A systematic review.J Oral Maxillofac Pathol. 2024 Jul-Sep;28(3):443-454. doi: 10.4103/jomfp.jomfp_511_23. Epub 2024 Oct 15. J Oral Maxillofac Pathol. 2024. PMID: 39670118 Free PMC article. Review.
-
Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19.J Korean Med Sci. 2023 Nov 13;38(44):e371. doi: 10.3346/jkms.2023.38.e371. J Korean Med Sci. 2023. PMID: 37967881 Free PMC article. Review.
References
-
- Joshi RK, Muralidharan CG, Gulati DS, Mopagar V, Dev JK, Kuthe S, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Medical journal, Armed Forces India. 2021;77(Suppl 2):S505–s7. - PMC - PubMed
-
- Bukhari MH, Syed M, Zain S. The Differences between Traditional Vaccines and RNA Vaccines: Safety, Efficacy, Reliability and Future of COVID-19 Vaccines. Annals of King Edward Medical University Lahore Pakistan. 2021;27(2):172–82.
LinkOut - more resources
Full Text Sources