Natural Killer Cell Expansion and Cytotoxicity Differ Depending on the Culture Medium Used
- PMID: 35765872
- PMCID: PMC9277036
- DOI: 10.3343/alm.2022.42.6.638
Natural Killer Cell Expansion and Cytotoxicity Differ Depending on the Culture Medium Used
Abstract
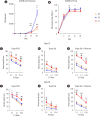
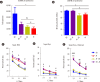
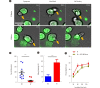
Background: Adoptive cell therapy using umbilical cord blood (UCB)-derived allogeneic natural killer (NK) cells has shown encouraging results. However, because of the insufficient availability of NK cells and limited UCB volume, more effective culture methods are required. NK cell expansion and functionality are largely affected by the culture medium. While human serum is a major affecting component in culture media, the way it regulates NK cell functionality remains elusive. We elucidated the effects of different culture media and human serum supplementation on UCB NK cell expansion and functionality.
Methods: UCB NK cells were cultured under stimulation with K562-OX40L-mbIL-18/21 feeder cells and IL-2 and IL-15 in serum-containing and serum-free culture media. The effects of the culture media and human serum supplementation on NK cell expansion and cytotoxicity were evaluated by analyzing the expansion rate, activating and inhibitory receptor levels, and the cytotoxicity of the UCB NK cells.
Results: The optimal medium for NK cell expansion was Dulbecco's modified Eagle's medium/Ham's F12 with supplements and that for cytotoxicity was AIM V supplemented with Immune Cell Serum Replacement. Shifting media is an advantageous strategy for obtaining several highly functional UCB NK cells. Live cell imaging and killing time measurement revealed that human serum enhanced NK cell proliferation but delayed target recognition, resulting in reduced cytotoxicity.
Conclusions: Culture medium supplementation with human serum strongly affects UCB NK cell expansion and functionality. Thus, culture media should be carefully selected to ensure both NK cell quantity and quality for adoptive cell therapy.
Keywords: Culture medium; Expansion; Feeder cells; Human serum; Natural killer cells; Umbilical cord blood.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the study reported in this paper.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

