The Pathogenic Yeast Candida parapsilosis Forms Pseudohyphae through Different Signaling Pathways Depending on the Available Carbon Source
- PMID: 35766504
- PMCID: PMC9241547
- DOI: 10.1128/msphere.00029-22
The Pathogenic Yeast Candida parapsilosis Forms Pseudohyphae through Different Signaling Pathways Depending on the Available Carbon Source
Abstract
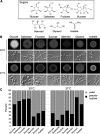
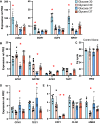
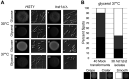
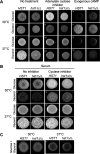
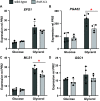
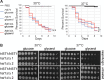
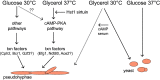
Candida parapsilosis is an emerging fungal pathogen that primarily affects immunocompromised patients in hospitals. A significant risk factor is the use of implanted medical devices, which support the growth of biofilms composed of a mixture of individual yeast cells and chains of elongated pseudohyphal cells. The morphological switch between these two forms is triggered by cues from the environment, including nutrient availability and temperature. We examined how different nutrient sources affect the balance between yeast and pseudohyphae and found that cells grown in the presence of five- or six-carbon sugars form more pseudohyphae at 30°C than at 37°C. Conversely, cells grown on glycerol, a three-carbon polyalcohol, form more pseudohyphae at 37°C. Furthermore, we found that different regulators influence pseudohyphal growth on glucose at 30°C compared with those on glycerol at 37°C. In particular, cAMP signaling and the sirtuin deacetylase Hst1 were required for pseudohyphal growth on glycerol at 37°C but not on glucose at 30°C. Finally, we found that the carbon source on which C. parapsilosis is grown can influence its ability to establish an infection in a wax moth model. Overall, this study reveals that environmental conditions affect not only the extent of pseudohyphal growth but also which pathways and regulators govern pseudohyphal formation. IMPORTANCE Candida parapsilosis is one of the leading causes of hospital-acquired yeast infections and poses a significant risk to immunocompromised people. Two of its properties that contribute to infection are metabolic flexibility, to use a range of nutrients available in the host, and cellular dimorphism, to switch between round yeast cells and chains of elongated pseudohyphal cells. Uncovering the molecular mechanisms that regulate these processes could reveal new targets for antifungal drugs. We found that for C. parapsilosis, the balance between yeast and pseudohyphal cells depends on the nutrients available and the growth temperature. Moreover, these environmental changes can affect its ability to cause infections. Finally, we found that a potential sensor of the cell's metabolic state, the sirtuin Hst1, contributes to pseudohyphal growth for cells grown on glycerol. These findings indicate that the shape and virulence of C. parapsilosis likely vary depending on its location in the host.
Keywords: Candida; cyclic AMP; filamentation; glycerol; sirtuin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Almirante B, Rodriguez D, Cuenca-Estrella M, Almela M, Sanchez F, Ayats J, Alonso-Tarres C, Rodriguez-Tudela JL, Pahissa A, the Barcelona Candidemia Project Study Group . 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 44:1681–1685. doi:10.1128/JCM.44.5.1681-1685.2006. - DOI - PMC - PubMed
-
- Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T, the National Nosocomial Infections Surveillance System Hospitals . 2006. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics 117:1680–1687. doi:10.1542/peds.2005-1996. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

