MBZM-N-IBT, a Novel Small Molecule, Restricts Chikungunya Virus Infection by Targeting nsP2 Protease Activity In Vitro, In Vivo, and Ex Vivo
- PMID: 35766508
- PMCID: PMC9295557
- DOI: 10.1128/aac.00463-22
MBZM-N-IBT, a Novel Small Molecule, Restricts Chikungunya Virus Infection by Targeting nsP2 Protease Activity In Vitro, In Vivo, and Ex Vivo
Abstract
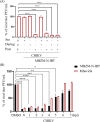
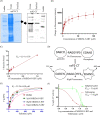
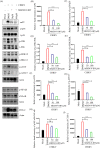
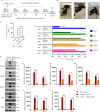
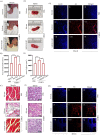
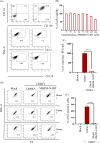
The increase in disease incidences and persistent Chikungunya virus (CHIKV)-induced arthritis have been a huge burden on public health globally. In the absence of specific antivirals or vaccines, it is essential to continue efforts to develop effective anti-CHIKV strategies. Our previous study showing the in vitro anti-CHIKV potential of a novel molecule 1-[(2-methylbenzimidazol-1-yl) methyl]-2-oxo-indolin-3-ylidene] amino] thiourea (MBZM-N-IBT) encouraged us to further validate its efficacy. Here, the effect of MBZM-N-IBT was evaluated in vitro in RAW 264.7 cells, in vivo in C57BL/6 mice, and ex vivo in human peripheral blood mononuclear cells (hPBMCs). The study demonstrated that CHIKV infection was efficiently abrogated in RAW 264.7 cells (IC50 = 22.34 μM) with significant inhibition in viral proteins. The inhibition was effective in the postentry step, and MBZM-N-IBT predominately interfered in the early stages of CHIKV life cycle. It was further supported when the protease activity of CHIKV-nsP2 was hindered by the compound. Moreover, it diminished the CHIKV-induced inflammatory responses in vitro through significant downregulation of all the major mitogen-activated protein kinases (MAPKs), NF-κB, cyclooxygenase (COX)-2, and cytokines. Furthermore, MBZM-N-IBT restricted CHIKV infection and inflammation in vivo, leading to reduced clinical scores and complete survival of C57BL/6 mice. Additionally, it has been noticed that the CHIKV infection was reduced remarkably in hPBMC-derived monocyte-macrophage populations ex vivo by the compound. In conclusion, it can be suggested that this novel compound MBZM-N-IBT has been demonstrated to be a potential anti-CHIKV molecule in vitro, in vivo, and ex vivo and fulfilled all the criteria to investigate further for successful treatment of CHIKV infection.
Keywords: Chikungunya; anti-viral; infection; inflammation; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. 2013. Chikungunya virus infection: an overview. New Microbiol 36:211–227. - PubMed
-
- Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, Kohl A, Rudd PA, Taylor A, Herrero LJ, Zaid A, Ng LFP, Mahalingam S. 2017. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis 17:e107–e117. 10.1016/S1473-3099(16)30385-1. - DOI - PubMed
-
- Mbaika S, Lutomiah J, Chepkorir E, Mulwa F, Khayeka-Wandabwa C, Tigoi C, Oyoo-Okoth E, Mutisya J, Ng'ang'a Z, Sang R. 2016. Vector competence of Aedes aegypti in transmitting chikungunya virus: effects and implications of extrinsic incubation temperature on dissemination and infection rates. Virol J 13:114. 10.1186/s12985-016-0566-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

