Melanoma-Specific Clinical Outcomes of Inpatient Immune Checkpoint Blockade Treatment
- PMID: 35766876
- PMCID: PMC9438908
- DOI: 10.1093/oncolo/oyac121
Melanoma-Specific Clinical Outcomes of Inpatient Immune Checkpoint Blockade Treatment
Abstract
Background: Little is known about patient outcomes with advanced melanoma following inpatient initiation or continuation of immune checkpoint blockade (ICB).
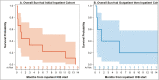
Methods and results: We conducted a single institution retrospective case series of advanced melanoma patients who initiated ICB as an inpatient (initial inpatient cohort, n = 9), or continued ICB as an inpatient after previously starting as an outpatient (outpatient then inpatient cohort, n = 5). One patient had a partial response to ICB initiated as an inpatient, but ultimately died of melanoma after 13.5 months. Median overall survival for initial inpatient cohort was 1.0 month (95% CI: 0.2-11.2), and 1.4 months (95% CI: 0.4-58.0) for the outpatient then inpatient cohort. Three patients were alive >6 months after inpatient ICB administration.
Conclusion: Despite overall poor outcomes, some patients may benefit from inpatient ICB. This study provides additional information for clinicians to appropriately counsel patients on expectations following inpatient ICB.
Keywords: immunotherapy; inpatient treatment; melanoma.
© The Author(s) 2022. Published by Oxford University Press.
Figures
References
-
- Wong A, Williams M, Milne D, et al. . Clinical and palliative care outcomes for patients of poor performance status treated with antiprogrammed death-1 monoclonal antibodies for advanced melanoma. Asia Pac J Clin Oncol. 2017;13(6):385-390. 10.1111/ajco.12702. 10.1111/ajco.12702. - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

