Envisioning clinical trials as complex interventions
- PMID: 35766902
- PMCID: PMC9378578
- DOI: 10.1002/cncr.34357
Envisioning clinical trials as complex interventions
Abstract
Clinical trials are critical components of modern health care and infrastructure. Trials benefit society through scientific advancement and individual patients through trial participation. In fact, billions of dollars are spent annually in support of these benefits. Despite the massive investments, clinical trials often fail to accomplish their primary aims and trial enrollment rates remain low. Prior efforts to improve trial conduct and enrollment have had limited success, perhaps due to oversimplification of the complex, multilevel nature of trials. For these reasons, the authors propose applying implementation science to the clinical trials context. In this commentary, the authors posit clinical trials as complex, multilevel evidence-based interventions with significant societal and individual benefits yet with persistent gaps in implementation. An application of implementation science concepts to the clinical trials context as means to build common vocabulary and establish a platform for applying implementation science and practice to improve clinical trial conduct is introduced. Applying implementation science to the clinical trials context can augment improvement efforts and build capacity for better and more efficient evidence-based care for all patients and trial stakeholders throughout the clinical trials enterprise.
Keywords: clinical trials; complex interventions; health services research; implementation science; quality improvement.
© 2022 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Laura J. Damschroder reports a grant (paid to her institution) from the VA Quality Enhancement Research Initiative Program (QUE 20–025); payments as an expert implementation science consultant on National Institutes of Health‐funded projects; honoraria from Dartmouth College, Intermountain Healthcare, New York University, Oregon Health Sciences University, University of Illinois, University of Chicago, University of Michigan, University of Zurich, Upstream USA Inc, Yale University, McGill University, Agenica de Qualitat I Avaluacio Sanitaries de Catalunya, Brown University, Karolinska Institutet, Northwell Health, Stanford University, University of Kentucky, University of Limerick, University of Pittsburgh, University of Wisconsin, University of Texas Health Sciences Center; and participation on a Data Safety Monitoring Board for National Institutes of Health/National Institute on Drug Abuse. Anne Sales is a co‐investigator on three grants from National Institutes of Health; has received honoraria for a lecture at the University of Kentucky; and is the Chair of the Data and Safety Monitoring Board for four asthma‐related projects for the National Heart, Lung, and Blood Institute. Ted Skolarus reports grants from the National Institutes of Health/National Cancer Institute (R01 CA242559 and R37 CA222885) and royalties from UpToDate. Kristian Stensland reports a grant from the National Institutes of Health/National Cancer Institute (T32 CA180984).
Figures
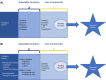
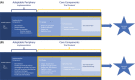
References
-
- Guidelines NCCN. NCCN clinical practice guidelines in oncology: prostate cancer. Published online February 2, 2021. Accessed February 5, 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
-
- May M. Clinical trial costs go under the microscope. Nat Med. Published online March 6, 2019. doi:10.1038/d41591-019-00008-7 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

