Clinical and radiological features of lung disorders related to connective-tissue diseases: a pictorial essay
- PMID: 35767157
- PMCID: PMC9243214
- DOI: 10.1186/s13244-022-01243-2
Clinical and radiological features of lung disorders related to connective-tissue diseases: a pictorial essay
Abstract
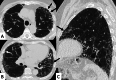
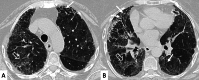
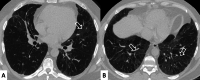
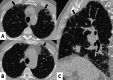
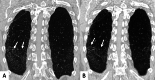
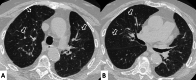
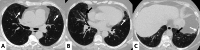
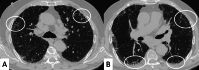
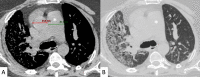
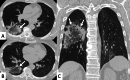
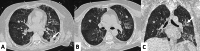
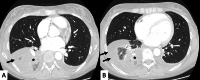
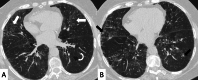
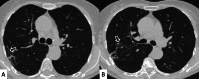
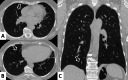
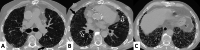
Connective tissue diseases (CTDs) include a spectrum of disorders that affect the connective tissue of the human body; they include autoimmune disorders characterized by immune-mediated chronic inflammation and the development of fibrosis. Lung involvement can be misdiagnosed, since pulmonary alterations preceded osteo-articular manifestations only in 20% of cases and they have no clear clinical findings in the early phases. All pulmonary structures may be interested: pulmonary interstitium, airways, pleura and respiratory muscles. Among these autoimmune disorders, rheumatoid arthritis (RA) is characterized by usual interstitial pneumonia (UIP), pulmonary nodules and airway disease with air-trapping, whereas non-specific interstitial pneumonia (NSIP), pulmonary hypertension and esophageal dilatation are frequently revealed in systemic sclerosis (SSc). NSIP and organizing pneumonia (OP) may be found in patients having polymyositis (PM) and dermatomyositis (DM); in some cases, perilobular consolidations and reverse halo-sign areas may be observed. Systemic lupus erythematosus (SLE) is characterized by serositis, acute lupus pneumonitis and alveolar hemorrhage. In the Sjögren syndrome (SS), the most frequent pattern encountered on HRCT images is represented by NSIP; UIP and lymphocytic interstitial pneumonia (LIP) are reported with a lower frequency. Finally, fibrotic NSIP may be the interstitial disease observed in patients having mixed connective tissue diseases (MCTD). This pictorial review therefore aims to provide clinical features and imaging findings associated with autoimmune CTDs, in order to help radiologists, pneumologists and rheumatologists in their diagnoses and management.
Keywords: Autoimmune diseases; Connective tissue disease; Lung disease (Interstitial); Multidetector computed tomography; Pulmonary fibrosis.
© 2022. The Author(s).
Conflict of interest statement
SP reports personal consulting fees and/or speaker fees from Boehringer Ingelheim and F. HoffmannLa Roche Ltd. outside the submitted work. GS reports personal fees from Boehringer Ingelheim outside the submitted work. CV is part of the F. Hoffmann-La Roche Ltd. and Boehringer Ingelheim Scientific board. He has received consulting fees and/or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, F. Hoffmann-La Roche Ltd and Menarini.
Figures














References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

