Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: a glucose-independent effect through AMPK signaling
- PMID: 35767208
- PMCID: PMC9325846
- DOI: 10.1007/s12272-022-01391-5
Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: a glucose-independent effect through AMPK signaling
Abstract
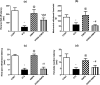
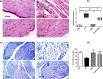
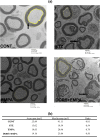
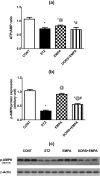
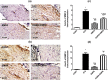
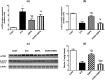
Diabetic peripheral neuropathy (DPN) represents a severe microvascular condition that dramatically affects diabetic patients despite adequate glycemic control, resulting in high morbidity. Thus, recently, anti-diabetic drugs that possess glucose-independent mechanisms attracted attention. This work aims to explore the potentiality of the selective sodium-glucose cotransporter-2 inhibitor, empagliflozin (EMPA), to ameliorate streptozotocin-induced DPN in rats with insight into its precise signaling mechanism. Rats were allocated into four groups, where control animals received vehicle daily for 2 weeks. In the remaining groups, DPN was elicited by single intraperitoneal injections of freshly prepared streptozotocin and nicotinamide (52.5 and 50 mg/kg, respectively). Then EMPA (3 mg/kg/p.o.) was given to two groups either alone or accompanied with the AMPK inhibitor dorsomorphin (0.2 mg/kg/i.p.). Despite the non-significant anti-hyperglycemic effect, EMPA improved sciatic nerve histopathological alterations, scoring, myelination, nerve fibers' count, and nerve conduction velocity. Moreover, EMPA alleviated responses to different nociceptive stimuli along with improved motor coordination. EMPA modulated ATP/AMP ratio, upregulated p-AMPK while reducing p-p38 MAPK expression, p-ERK1/2 and consequently p-NF-κB p65 as well as its downstream mediators (TNF-α and IL-1β), besides enhancing SOD activity and lowering MDA content. Moreover, EMPA downregulated mTOR and stimulated ULK1 as well as beclin-1. Likewise, EMPA reduced miR-21 that enhanced RECK, reducing MMP-2 and -9 contents. EMPA's beneficial effects were almost abolished by dorsomorphin administration. In conclusion, EMPA displayed a protective effect against DPN independently from its anti-hyperglycemic effect, probably via modulating the AMPK pathway to modulate oxidative and inflammatory burden, extracellular matrix remodeling, and autophagy.
Keywords: AMPK; Diabetic peripheral neuropathy; Dorsomorphin; Empagliflozin; mTOR; p38 MAPK.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no Competing interest.
Figures


References
-
- Abdelhamid AM, Youssef ME, Abd El-Fattah EE, Gobba NA, Gaafar AGA, Girgis S, Shata A, Hafez AM, El-Ahwany E, Amin NA, Shahien MA, Abd-Eldayem MA, Abou-Elrous M, Saber S. Blunting p38 MAPKα and ERK1/2 activities by empagliflozin enhances the antifibrotic effect of metformin and augments its AMPK-induced NF-κB inactivation in mice intoxicated with carbon tetrachloride. Life Sci. 2021;286:120070. doi: 10.1016/j.lfs.2021.120070. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

