Absolute and Relative Risk of New-Onset Psoriasis Associated With Tumor Necrosis Factor-α Inhibitor Treatment in Patients With Immune-Mediated Inflammatory Diseases: A Danish Nationwide Cohort Study
- PMID: 35767240
- PMCID: PMC9244773
- DOI: 10.1001/jamadermatol.2022.2360
Absolute and Relative Risk of New-Onset Psoriasis Associated With Tumor Necrosis Factor-α Inhibitor Treatment in Patients With Immune-Mediated Inflammatory Diseases: A Danish Nationwide Cohort Study
Abstract
Importance: Tumor necrosis factor-α inhibitor (TNFi)-associated psoriasis is a rare adverse event following TNFi treatment. Data on the risk of developing TNFi-associated psoriasis when treated with TNFi are sparse.
Objective: To investigate the associated risk between new-onset psoriasis and TNFi treatment compared with nonbiologic conventional treatment.
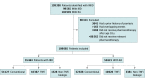
Design, setting, and participants: Using Danish national registries (1995-2018), this cohort study included patients with inflammatory bowel disease (IBD) and/or rheumatoid arthritis (RA) who received either conventional therapy or TNFi treatment. Patients may not have been diagnosed with psoriasis prior to initiation of treatment. Patients were followed up for up to 5 years. Cox regression models with robust variance were used to compare the risk of developing any type of psoriasis, nonpustular psoriasis, and pustular psoriasis. Patients receiving conventional therapy were used as reference. Data analysis was performed from January 1995 to December 2018.
Exposures: For the present study, the term conventional therapy was used for the nonbiological therapy. For biological therapy, a distinction was made between TNFi treatment and non-TNFi biological therapy.
Main outcomes and measures: The outcome of psoriasis was defined as a registered International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code of psoriasis and/or having 2 consecutive prescriptions of topical vitamin D analogues.
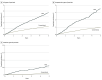
Results: The study included 109 085 patients, of which 62% were female. Median (IQR) age was 50 (34-64) years. Of the included patients, 108 024 received conventional therapy and 20 910 received TNFi treatment. During follow-up, 1471 (1.4%) patients developed any type of psoriasis, of which 1332 developed nonpustular psoriasis, 127 patients developed palmoplantar pustulosis, and 12 patients developed generalized pustulosis. The incidence rates for developing any type of psoriasis per 1000 patient-years were 3.0 (95% CI, 2.9-3.2) for conventional therapy and 7.8 (95% CI, 7.5-8.9) for TNFi. During treatment with TNFi, the hazard ratio was 2.12 (95% CI, 1.87-2.40; P < .001) for developing nonpustular psoriasis and 6.50 (95% CI, 4.60-9.23; P < .001) for pustular psoriasis compared with conventional treatment. Exposure needed for 1 additional patient to be harmed was 241 patient-years for any type of TNFi-associated psoriasis, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis.
Conclusions and relevance: In a Danish nationwide cohort of patients with immune-mediated inflammatory diseases treated with TFNi or conventional treatment and no history of psoriasis, in TFNi-treated patients, nonpustular types of psoriasis constituted the most events, whereas pustular types of psoriasis had the highest relative risk. Although the risk of new-onset psoriasis increased for both nonpustular and pustular types of psoriasis in TFNi-treated patients, the absolute risk remained modest at 241 patient-years of exposure need for 1 additional event and an estimated absolute risk difference around 5 per 1000 patient-years, indicating that the approach to treatment of patients in need of TNFi treatment should not change.
Conflict of interest statement
Figures

Comment in
-
Tumor necrosis factor-α inhibitor-associated psoriasis: Facts and hypotheses.Int J Rheum Dis. 2023 Jun;26(6):1011-1014. doi: 10.1111/1756-185X.14661. Int J Rheum Dis. 2023. PMID: 37306678 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

