Risk of Hepatocellular Carcinoma With Tenofovir vs Entecavir Treatment for Chronic Hepatitis B Virus: A Reconstructed Individual Patient Data Meta-analysis
- PMID: 35767258
- PMCID: PMC9244612
- DOI: 10.1001/jamanetworkopen.2022.19407
Risk of Hepatocellular Carcinoma With Tenofovir vs Entecavir Treatment for Chronic Hepatitis B Virus: A Reconstructed Individual Patient Data Meta-analysis
Abstract
Importance: Conventional meta-analyses with aggregated study-level data have yielded conflicting results for the comparative effectiveness of tenofovir disoproxil fumarate vs entecavir in reducing hepatocellular carcinoma (HCC) risk among patients with chronic hepatitis B virus. Within-study heterogeneity, between-study heterogeneity, and the inability of conventional meta-analyses to capture time-to-event data were associated with these results.
Objective: To perform a reconstructed individual patient data meta-analysis of high-quality propensity score-matched studies to provide robust estimates for comparative HCC risk between groups receiving tenofovir or entecavir.
Data sources: Medline and Embase databases were searched from inception to October 6, 2021.
Study selection: The initial search yielded 3435 articles. Fourteen studies that used propensity score matching to balance baseline characteristics were included in the final analysis.
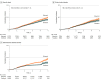
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline was followed. Individual patient data were reconstructed from Kaplan-Meier curves. Risk of HCC was evaluated using random-effects hazard ratios (HRs) via a shared-frailty model and a Cox proportional hazards model stratified by study group. Restricted mean survival time (RMST) analysis was conducted to account for varying estimated treatment effect across time.
Main outcomes and measures: The comparative risk of HCC with tenofovir vs entecavir treatment.
Results: From analysis of 14 studes with 24 269 patients (10 534 receiving tenofovir and 13 735 receiving entecavir; mean age, 49.86 [95% CI, 48.35-51.36] years; 65.05% [95% CI, 58.60%-71.00%] men), tenofovir was associated with decreased HCC incidence compared with entecavir (stratified Cox HR, 0.85 [95% CI, 0.76-0.94] at 5 years; P = .002). However, there was no significant difference in subanalysis of clinical cohort studies (stratified Cox HR, 0.92 [95% CI, 0.80-1.06] at 5 years; P = .24). Among administrative database studies, proportionality was violated, and HRs could not be obtained via Cox proporational hazards-based models. The mean time to HCC development in RMST analysis was 2.8 (95% CI, 1.8-3.7) weeks longer (P < .001) for tenofovir vs entecavir at 5 years. The RMST analyses for other subgroups revealed either insignificant or minimal differences (<3 weeks) in the mean time to HCC at 5 years.
Conclusions and relevance: In this meta-analysis, there was no clinically meaningful difference in the risk of HCC between patients who received entecavir and patients who received tenofovir. There was no difference between tenofovir and entecavir among clinical cohort studies, whereas the mean time to HCC development was less than 3 weeks longer for patients who received tenofovir vs those who received entecavir at year 5 among administrative database studies. The choice between tenofovir or entecavir should be decided based on patient convenience and tolerability.
Conflict of interest statement
Figures
References
-
- World Health Organization Global Hepatitis Report . 2017. Accessed May 20, 2020. https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-en...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical