Prognostic attributes of immune signatures in soft tissue sarcomas show differential dependencies on tumor mutational burden
- PMID: 35767280
- PMCID: PMC9544607
- DOI: 10.1002/cncr.34333
Prognostic attributes of immune signatures in soft tissue sarcomas show differential dependencies on tumor mutational burden
Abstract
Background: Cellular and intrinsic markers of sarcoma immunogenicity are poorly understood. To gain insight into whether tumor-immune interactions correlate with clinical aggressiveness, the authors examined the prognostic significance of immune gene signatures in combination with tumor mutational burden (TMB) and cancer-testis antigen (CTA) expression.
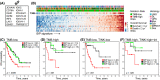
Methods: RNA sequencing and clinical data of 259 soft tissue sarcomas from The Cancer Genome Atlas project were used to investigate associations between published immune gene signatures and patient overall survival (OS) in the contexts of TMB, as computed from whole-exome sequencing data, and CTA gene expression. Multivariate Cox proportional hazards regression models and log-rank tests were used to assess survival associations.
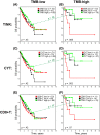
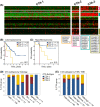
Results: Immune signature scores that reflected in part the intratumoral abundance of cytotoxic T cells showed significant positive associations with OS. However, the prognostic power of the T-cell signatures was highly dependent on TMB-high status, consistent with protective effects of tumor-infiltrating T cells in tumors with elevated antigenicity. In TMB-low tumors, a signature of infiltrating plasma B cells was significantly and positively associated with OS, independent of T-cell signature status. Although tumor subtypes based on differential expression patterns of CTA genes showed different survival associations within leiomyosarcoma and myxofibrosarcoma histologies, neither CTA nor histologic subtype interacted with the T-cell-survival association.
Conclusions: Signatures of T-cell and plasma B-cell infiltrates were associated with a survival benefit in soft tissue sarcomas. TMB, but not CTA expression, influenced the prognostic power of T-cell-associated, but not plasma B-cell-associated, survival.
Lay summary: Clinical data and RNA analysis of 259 soft tissue sarcomas from The Cancer Genome Atlas project were used to investigate associations between five published gene immune cell expression signatures and survival in the context of tumor mutations. Activated T cells had a significant positive association with patient survival. Although high tumor mutation burden was associated with good survival, the prognostic power of T-cell signatures was highly dependent on tumor mutational status, consistent with protective effects of tumor-infiltrating T cells in tumors with high levels of antigens. In low tumor mutation-bearing tumors, plasma B cells were positively associated with survival.
Keywords: burden; immune; mutational; prognosis; signatures; tumor.
© 2022 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
The authors made no disclosures.
Figures


References
-
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415‐423. - PubMed
-
- Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a phase III, placebo‐controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34:3898‐3905. - PubMed
-
- Tsung K, Norton JA. Lessons from Coley's Toxin. Surg Oncol. 2006;15:25‐28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

