A phase IB/IIA study of remestemcel-L, an allogeneic bone marrow-derived mesenchymal stem cell product, for the treatment of medically refractory ulcerative colitis: an interim analysis
- PMID: 35767384
- PMCID: PMC9795998
- DOI: 10.1111/codi.16239
A phase IB/IIA study of remestemcel-L, an allogeneic bone marrow-derived mesenchymal stem cell product, for the treatment of medically refractory ulcerative colitis: an interim analysis
Abstract
Aim: There have been no studies into the direct injection of mesenchymal stem cells (MSCs) for luminal ulcerative colitis (UC). Our aim was to investigate the efficacy of MSCs delivered locally via endoscopic delivery, as is done in the setting of perianal disease, to treat the local site of inflammation directly.
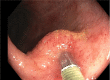
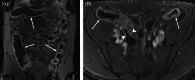
Method: A phase IB/IIA randomized control clinical trial of remestemcel-L, an ex vivo expanded allogeneic bone marrow-derived MSC product, at a dose of 150 million MSCs versus placebo (2:1 fashion) delivered via direct injection using a 23-gauge sclerotherapy needle at the time of colonoscopy was designed to assess the safety and efficacy of endoscopic delivery of MSCs for UC. The main outcome measures were adverse events, Mayo score and Mayo endoscopic severity score at 2 weeks, 6 weeks and 3 months post-MSC delivery.
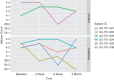
Results: Six patients were enrolled and treated; four received MSCs and two placebo. All had been on prior anti-tumour necrosis factor or anti-integrin therapy. There were no adverse events related to MSCs. In the treatment group (n = 4), the Mayo endoscopic severity score decreased in all patients by 2 weeks after MSC delivery. At 3 months, all patients were extremely satisfied or satisfied with their MSC treatment based on the inflammatory bowel disease patient-reported treatment impact (IBD-PRTI), and treatment response was described as excellent or good in all patients. In the control group (n = 2), the Mayo endoscopic severity score did not increase as a result of being off alternative therapy. At 3 months, patients were dissatisfied according to the IBD-PRTI, and treatment response was poor or unchanged.
Conclusion: MSCs may offer a safe therapeutic option for the treatment of medically refractory UC. Early data suggest improved clinical and endoscopic scores by 2 weeks after MSC delivery.
Keywords: Mayo score; mesenchymal stem cells; ulcerative colitis.
© 2022 The Authors. Colorectal Disease published by John Wiley & Sons Ltd on behalf of Association of Coloproctology of Great Britain and Ireland.
Conflict of interest statement
ALL is a consultant for Takeda, Mesoblast and Ossium.
Figures




Similar articles
-
A Phase IB/IIA Study of Allogeneic, Bone Marrow-derived, Mesenchymal Stem Cells for the Treatment of Refractory Ileal-anal Anastomosis and Peripouch Fistulas in the Setting of Crohn's Disease of the Pouch.J Crohns Colitis. 2023 Apr 19;17(4):480-488. doi: 10.1093/ecco-jcc/jjac172. J Crohns Colitis. 2023. PMID: 36322714 Clinical Trial.
-
[Allogeneic mesenchymal stromal cells in patients with ulcerative colitis: two years of observation].Eksp Klin Gastroenterol. 2010;(11):3-15. Eksp Klin Gastroenterol. 2010. PMID: 21485508 Clinical Trial. Russian.
-
Remestemcel-L allogeneic bone marrow-derived mesenchymal stem cell product to treat medically refractory Crohn's colitis: preliminary phase IB/IIA study.Br J Surg. 2022 Jul 15;109(8):653-655. doi: 10.1093/bjs/znac078. Br J Surg. 2022. PMID: 35714218 Clinical Trial. No abstract available.
-
Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta-analysis of experimental and clinical studies.Stem Cell Res Ther. 2019 Aug 23;10(1):266. doi: 10.1186/s13287-019-1336-4. Stem Cell Res Ther. 2019. PMID: 31443677 Free PMC article.
-
The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer.Stem Cell Res Ther. 2022 Apr 1;13(1):138. doi: 10.1186/s13287-022-02811-5. Stem Cell Res Ther. 2022. PMID: 35365226 Free PMC article. Review.
Cited by
-
Reconstitution of T cell-mediated immunity by umbilical cord-derived mesenchymal stem cells in ulcerative colitis.Clin Transl Med. 2025 Aug;15(8):e70452. doi: 10.1002/ctm2.70452. Clin Transl Med. 2025. PMID: 40842289 Free PMC article.
-
Integration and implementation of precision medicine in the multifaceted inflammatory bowel disease.World J Gastroenterol. 2023 Sep 28;29(36):5211-5225. doi: 10.3748/wjg.v29.i36.5211. World J Gastroenterol. 2023. PMID: 37901450 Free PMC article. Review.
-
Mesenchymal Stem Cells Ameliorate DSS-Induced Experimental Colitis by Modulating the Gut Microbiota and MUC-1 Pathway.J Inflamm Res. 2023 May 11;16:2023-2039. doi: 10.2147/JIR.S402592. eCollection 2023. J Inflamm Res. 2023. PMID: 37197438 Free PMC article.
-
Immunomodulatory Mechanism and Potential Application of Dental Pulp-Derived Stem Cells in Immune-Mediated Diseases.Int J Mol Sci. 2023 Apr 29;24(9):8068. doi: 10.3390/ijms24098068. Int J Mol Sci. 2023. PMID: 37175774 Free PMC article. Review.
-
The Impact of Cellular Therapies on Gastrointestinal Diseases: Applications and Challenges.Turk J Gastroenterol. 2023 Aug;34(8):782-794. doi: 10.5152/tjg.2023.23137. Turk J Gastroenterol. 2023. PMID: 37485563 Free PMC article. Review.
References
-
- Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182–97. - PubMed
-
- Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non‐response to tumor necrosis factor antagonists is associated with inferior response to second‐line biologics in patients with inflammatory bowel diseases: a systematic review and meta‐analysis. J Crohns Colitis. 2018;12(6):635–43. - PMC - PubMed
-
- Kin C, Kate Bundorf M. As infliximab use for ulcerative colitis has increased, so has the rate of surgical resection. J Gastrointest Surg. 2017;21(7):1159–65. - PubMed
-
- Garcia‐Olmo D, Garcia‐Arranz M, Garcia LG, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell‐based therapy. Int J Colorectal Dis. 2003;18(5):451–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

