Primary Aldosteronism: State-of-the-Art Review
- PMID: 35767459
- PMCID: PMC9729786
- DOI: 10.1093/ajh/hpac079
Primary Aldosteronism: State-of-the-Art Review
Abstract
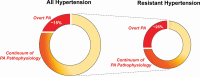
We are witnessing a revolution in our understanding of primary aldosteronism (PA). In the past 2 decades, we have learned that PA is a highly prevalent syndrome that is largely attributable to pathogenic somatic mutations, that contributes to cardiovascular, metabolic, and kidney disease, and that when recognized, can be adequately treated with widely available mineralocorticoid receptor antagonists and/or surgical adrenalectomy. Unfortunately, PA is rarely diagnosed, or adequately treated, mainly because of a lack of awareness and education. Most clinicians still possess an outdated understanding of PA; from primary care physicians to hypertension specialists, there is an urgent need to redefine and reintroduce PA to clinicians with a modern and practical approach. In this state-of-the-art review, we provide readers with the most updated knowledge on the pathogenesis, prevalence, diagnosis, and treatment of PA. In particular, we underscore the public health importance of promptly recognizing and treating PA and provide pragmatic solutions to modify clinical practices to achieve this.
Keywords: adrenal; aldosterone; blood pressure; hypertension; primary aldosteronism; renin.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Journal of Hypertension, Ltd. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Zennaro MC, Boulkroun S, Fernandes-Rosa F. Genetic causes of functional adrenocortical adenomas. Endocr Rev 2017; 38:516–537. - PubMed
-
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 2010; 95:2296–2305. - PubMed
-
- Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A, Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab 2013; 98:1567–1574. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

