Exploring solid-phase proximity ligation assay for survivin detection in urine
- PMID: 35767525
- PMCID: PMC9242480
- DOI: 10.1371/journal.pone.0270535
Exploring solid-phase proximity ligation assay for survivin detection in urine
Abstract
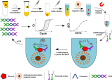
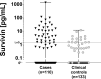
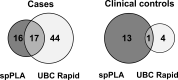
Urine-based biomarkers are a rational and promising approach for the detection of bladder cancer due to the proximity of urine to the location of the tumor site and the non-invasive nature of its sampling. A well-known and highly investigated biomarker for bladder cancer is survivin. For detection of very small amounts of urinary survivin protein a highly sensitive assay was developed. The assay is based on the immuno-PCR technology, more precisely a solid-phase proximity ligation assay (spPLA). The limit of detection for the survivin spPLA was 1.45 pg/mL, resulting in an improvement of the limit of detection by a factor of approximately 23 compared to the previously in-house developed survivin ELISA. A key step in development was the initial isolation of survivin by a molecular fishing rod based on magnetic beads. Interfering matrix compounds pose a special challenge for further analytical application, but can be overcome by this isolation step. The assay is designed to work with only 500 μL of voided urine. The survivin spPLA showed a sensitivity of 30% and specificity of 89% for bladder cancer detection in this study of 110 bladder cancer cases and 133 clinical controls. Moreover, the results demonstrated again that survivin is a useful complementary marker in combination with UBC® Rapid by increasing the overall sensitivity to 70% with a specificity of 86%. Although the performance for detection of bladder cancer was rather low, the herein developed assay might serve as a new tool for survivin biomarker research in diverse human fluids, even if the biological matrix is complex or survivin is only present in small amounts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
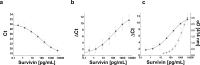
Similar articles
-
Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine.Int J Mol Sci. 2018 Jan 11;19(1):226. doi: 10.3390/ijms19010226. Int J Mol Sci. 2018. PMID: 29324722 Free PMC article.
-
The value of combined use of survivin mRNA and matrix metalloproteinase 2 and 9 for bladder cancer detection in voided urine.Dis Markers. 2013;34(1):57-62. doi: 10.3233/DMA-2012-0923. Dis Markers. 2013. PMID: 22960341 Free PMC article.
-
Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer.J Urol. 2004 Feb;171(2 Pt 1):626-30. doi: 10.1097/01.ju.0000107826.78479.90. J Urol. 2004. PMID: 14713774
-
Bladder cancer detection with urinary survivin, an inhibitor of apoptosis.Front Biosci. 2002 Feb 1;7:e36-41. doi: 10.2741/sharp. Front Biosci. 2002. PMID: 11815300 Review.
-
Survivin: role in diagnosis, prognosis, and treatment of bladder cancer.Adv Anat Pathol. 2006 May;13(3):122-6. doi: 10.1097/00125480-200605000-00003. Adv Anat Pathol. 2006. PMID: 16778475 Review.
Cited by
-
Comparison of Survivin Determination by Surface-Enhanced Fluorescence and Raman Spectroscopy on Nanostructured Silver Substrates.Biosensors (Basel). 2024 Oct 6;14(10):479. doi: 10.3390/bios14100479. Biosensors (Basel). 2024. PMID: 39451692 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

