Development of a carotid artery thrombolysis stroke model in mice
- PMID: 35767737
- PMCID: PMC9631707
- DOI: 10.1182/bloodadvances.2021006008
Development of a carotid artery thrombolysis stroke model in mice
Abstract
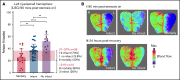
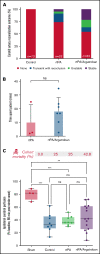
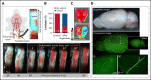
Recanalization with restored cerebral perfusion is the primary goal of thrombolytic therapy in acute ischemic stroke. The identification of adjunctive therapies that can be safely used to enhance thrombolysis in stroke remains an elusive goal. We report here the development of a mouse in situ carotid artery thrombolysis (iCAT) stroke model involving graded cerebral ischemia to induce unihemispheric infarction after thrombotic occlusion of the common carotid artery (CCA). Electrolytic-induced thrombotic occlusion of the left CCA enabled real-time assessment of recanalization and rethrombosis events after thrombolysis with recombinant tissue-type plasminogen activator (rtPA). Concurrent transient stenosis of the right CCA induced unihemispheric hypoperfusion and infarction in the left middle cerebral artery territory. Real-time assessment of thrombolysis revealed recanalization rates <30% in rtPA-treated animals with high rates of rethrombosis. Addition of the direct thrombin inhibitor argatroban increased recanalization rates to 50% and reduced rethrombosis. Paradoxically, this was associated with increased cerebral ischemia and stroke-related mortality (25%-42%). Serial analysis of carotid and cerebral blood flow showed that coadministration of argatroban with rtPA resulted in a marked increase in carotid artery embolization, leading to distal obstruction of the middle cerebral artery. Real-time imaging of carotid thrombi revealed that adjunctive anticoagulation destabilized platelet-rich thrombi at the vessel wall, leading to dislodgement of large platelet emboli. These studies confirm the benefits of anticoagulants in enhancing thrombolysis and large artery recanalization; however, at high levels of anticoagulation (∼3-fold prolongation of activated partial thromboplastin time), this effect is offset by increased incidence of carotid artery embolization and distal middle cerebral artery occlusion. The iCAT stroke model should provide important new insight into the effects of adjunctive antithrombotic agents on real-time thrombus dynamics during thrombolysis and their correlation with stroke outcomes.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures





References
-
- Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967-973. - PubMed
-
- National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1588. - PubMed
-
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6): 862-867. - PubMed
-
- Rubiera M, Alvarez-Sabín J, Ribo M, et al. . Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2005;36(7):1452-1456. - PubMed
-
- Saqqur M, Uchino K, Demchuk AM, et al. ; CLOTBUST Investigators . Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38(3):948-954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

