Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes
- PMID: 35767918
- PMCID: PMC9241134
- DOI: 10.1016/j.redox.2022.102382
Farnesoid X receptor protects against cisplatin-induced acute kidney injury by regulating the transcription of ferroptosis-related genes
Abstract
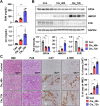
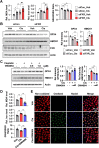
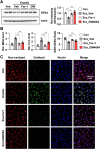
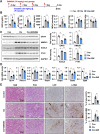
The side effects of cisplatin, a widely used chemotherapeutic agent, include nephrotoxicity. Previous studies have reported that cisplatin induces ferroptosis and lipid peroxide accumulation. Ferroptosis, a type of regulated cell death, is characterized by iron-dependent lipid peroxidation. Although previous studies have examined the regulation of ferroptosis in acute kidney injury (AKI), the regulatory mechanism of ferroptosis has not been elucidated. Here, the ability of activated farnesoid X receptor (FXR) to attenuate cisplatin-induced AKI through the regulation of ferroptosis was examined. FXR deficiency exhibited more ferroptosis responses, such as increase in lipid peroxidation, iron content and heme oxygenase 1 protein, and a decrease in glutathione/glutathione disulfide ratio and glutathione peroxidase 4 levels in HK2 cells and mice. Increased blood urea nitrogen, serum creatinine, and ferroptotic responses in the cisplatin-induced AKI mouse model were mitigated upon treatment with the FXR agonist GW4064 but were exacerbated in FXR knockout mice. RNA sequencing analysis revealed that ferroptosis-associated genes were novel targets of FXR. FXR agonist upregulated the expression of lipid and glutathione metabolism-related genes and downregulated cell death-related genes. Additionally, chromatin immunoprecipitation assays, using mice renal tissues, revealed that agonist-activated FXR could bind to its known target genes (Slc51a, Slc51b, Osgin1, and Mafg) and ferroptosis-related genes (Aifm2, Ggt6, and Gsta4). Furthermore, activated FXR-dependent MAFG, a transcriptional repressor, could bind to Hmox1, Nqo1, and Tf in the renal tissues of FXR agonist-treated mice. These findings indicate that activated FXR regulates the transcription of ferroptosis-related genes and protects against cisplatin-induced AKI.
Keywords: Acute kidney injury (AKI); Farnesoid X receptor (FXR); Ferroptosis; MAF bZIP transcription factor G (MAFG); Reactive oxidative stress (ROS).
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

