Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation
- PMID: 35768006
- PMCID: PMC9189143
- DOI: 10.1016/j.cell.2022.06.008
Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation
Abstract
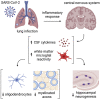
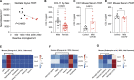
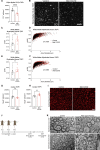
COVID survivors frequently experience lingering neurological symptoms that resemble cancer-therapy-related cognitive impairment, a syndrome for which white matter microglial reactivity and consequent neural dysregulation is central. Here, we explored the neurobiological effects of respiratory SARS-CoV-2 infection and found white-matter-selective microglial reactivity in mice and humans. Following mild respiratory COVID in mice, persistently impaired hippocampal neurogenesis, decreased oligodendrocytes, and myelin loss were evident together with elevated CSF cytokines/chemokines including CCL11. Systemic CCL11 administration specifically caused hippocampal microglial reactivity and impaired neurogenesis. Concordantly, humans with lasting cognitive symptoms post-COVID exhibit elevated CCL11 levels. Compared with SARS-CoV-2, mild respiratory influenza in mice caused similar patterns of white-matter-selective microglial reactivity, oligodendrocyte loss, impaired neurogenesis, and elevated CCL11 at early time points, but after influenza, only elevated CCL11 and hippocampal pathology persisted. These findings illustrate similar neuropathophysiology after cancer therapy and respiratory SARS-CoV-2 infection which may contribute to cognitive impairment following even mild COVID.
Keywords: COVID-19; H1N1 influenza; cognitive impairment; hippocampal neurogenesis; long COVID; microglia; myelin; neuroinflammation; oligodendrocytes.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.I. served as a consultant for Spring Discovery, Boehringer Ingelheim (Ingelheim, Germany), and Adaptive Biotechnologies. M.M. serves in the scientific advisory board of Cygnal Therapeutics. S.A.L. sits on the scientific advisory board and has a financial interest in AstronauTx Ltd. The opinions and assertions expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Figures



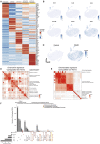
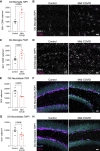
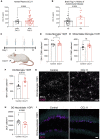
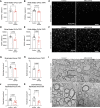


Comment in
-
Inflammation links mild COVID-19 with long-term cognitive impairment.Nat Rev Neurol. 2022 Aug;18(8):453. doi: 10.1038/s41582-022-00694-x. Nat Rev Neurol. 2022. PMID: 35773475 Free PMC article.
References
-
- Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. - DOI - PMC - PubMed
-
- Ayoub R., Ruddy R.M., Cox E., Oyefiade A., Derkach D., Laughlin S., Ades-Aron B., Shirzadi Z., Fieremans E., MacIntosh B.J., et al. Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat. Med. 2020;26:1285–1294. doi: 10.1038/s41591-020-0985-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EY033353/EY/NEI NIH HHS/United States
- T32 GM145402/GM/NIGMS NIH HHS/United States
- DP1 NS111132/NS/NINDS NIH HHS/United States
- R01 NS092597/NS/NINDS NIH HHS/United States
- K23 NS109284/NS/NINDS NIH HHS/United States
- P30 CA124435/CA/NCI NIH HHS/United States
- CRUK_/Cancer Research UK/United Kingdom
- T32 GM007365/GM/NIGMS NIH HHS/United States
- T32 AI007019/AI/NIAID NIH HHS/United States
- T32 CA009302/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K08 AI163493/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI157488/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

