Host lung microbiota promotes malaria-associated acute respiratory distress syndrome
- PMID: 35768411
- PMCID: PMC9243033
- DOI: 10.1038/s41467-022-31301-8
Host lung microbiota promotes malaria-associated acute respiratory distress syndrome
Abstract
Severe malaria can manifest itself with a variety of well-recognized clinical phenotypes that are highly predictive of death - severe anaemia, coma (cerebral malaria), multiple organ failure, and respiratory distress. The reasons why an infected individual develops one pathology rather than another remain poorly understood. Here we use distinct rodent models of infection to show that the host microbiota is a contributing factor for the development of respiratory distress syndrome and host mortality in the context of malaria infections (malaria-associated acute respiratory distress syndrome, MA-ARDS). We show that parasite sequestration in the lung results in sustained immune activation. Subsequent production of the anti-inflammatory cytokine IL-10 by T cells compromises microbial control, leading to severe lung disease. Notably, bacterial clearance with linezolid, an antibiotic commonly used in the clinical setting to control lung-associated bacterial infections, prevents MA-ARDS-associated lethality. Thus, we propose that the host's anti-inflammatory response to limit tissue damage can result in loss of microbial control, which promotes MA-ARDS. This must be considered when intervening against life-threatening respiratory complications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


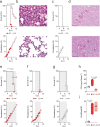
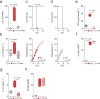

References
-
- World Health Organization (WHO), World Malaria Report (2021).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

