Natural killer cell homing and trafficking in tissues and tumors: from biology to application
- PMID: 35768424
- PMCID: PMC9243142
- DOI: 10.1038/s41392-022-01058-z
Natural killer cell homing and trafficking in tissues and tumors: from biology to application
Abstract
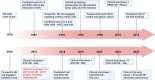
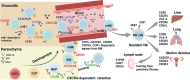
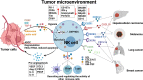
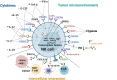
Natural killer (NK) cells, a subgroup of innate lymphoid cells, act as the first line of defense against cancer. Although some evidence shows that NK cells can develop in secondary lymphoid tissues, NK cells develop mainly in the bone marrow (BM) and egress into the blood circulation when they mature. They then migrate to and settle down in peripheral tissues, though some special subsets home back into the BM or secondary lymphoid organs. Owing to its success in allogeneic adoptive transfer for cancer treatment and its "off-the-shelf" potential, NK cell-based immunotherapy is attracting increasing attention in the treatment of various cancers. However, insufficient infiltration of adoptively transferred NK cells limits clinical utility, especially for solid tumors. Expansion of NK cells or engineered chimeric antigen receptor (CAR) NK cells ex vivo prior to adoptive transfer by using various cytokines alters the profiles of chemokine receptors, which affects the infiltration of transferred NK cells into tumor tissue. Several factors control NK cell trafficking and homing, including cell-intrinsic factors (e.g., transcriptional factors), cell-extrinsic factors (e.g., integrins, selectins, chemokines and their corresponding receptors, signals induced by cytokines, sphingosine-1-phosphate (S1P), etc.), and the cellular microenvironment. Here, we summarize the profiles and mechanisms of NK cell homing and trafficking at steady state and during tumor development, aiming to improve NK cell-based cancer immunotherapy.
© 2022. The Author(s).
Conflict of interest statement
J.Y. is a cofounder of CytoImmune Therapeutics, Inc. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

