Evaluating utility and feasibility of mismatch repair testing of colorectal cancer patients in a low-middle-income country
- PMID: 35768447
- PMCID: PMC9243080
- DOI: 10.1038/s41598-022-14644-6
Evaluating utility and feasibility of mismatch repair testing of colorectal cancer patients in a low-middle-income country
Abstract
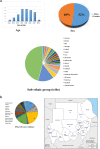
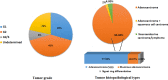
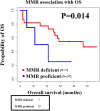
Molecular pathology services for colorectal cancer (CRC) in Sudan represent a significant unmet clinical need. In a retrospective cohort study involving 50 patients diagnosed with CRC at three major medical settings in Sudan, we aimed to outline the introduction of a molecular genetic service for CRC in Sudan, and to explore the CRC molecular features and their relationship to patient survival and clinicopathological characteristics. Mismatch repair (MMR) and BRAF (V600E) mutation status were determined by immunohistochemistry. A mismatch repair deficient (dMMR) subtype was demonstrated in 16% of cases, and a presumptive Lynch Syndrome (LS) diagnosis was made in up to 14% of patients. dMMR CRC in Sudan is characterized by younger age at diagnosis and a higher incidence of right-sided tumours. We report a high mortality in Sudanese CRC patients, which correlates with advanced disease stage, and MMR status. Routine MMR immunohistochemistry (with sequential BRAF mutation analysis) is a feasible CRC prognostic and predictive molecular biomarker, as well as a screening tool for LS in low-middle-income countries (LMICs).
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

