YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING
- PMID: 35768505
- PMCID: PMC7613988
- DOI: 10.1038/s41586-022-04924-6
YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING
Abstract
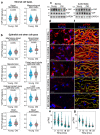
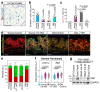
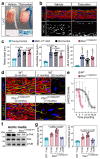
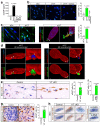
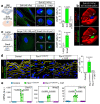
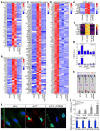
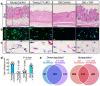
Ageing is intimately connected to the induction of cell senescence1,2, but why this is so remains poorly understood. A key challenge is the identification of pathways that normally suppress senescence, are lost during ageing and are functionally relevant to oppose ageing3. Here we connected the structural and functional decline of ageing tissues to attenuated function of the master effectors of cellular mechanosignalling YAP and TAZ. YAP/TAZ activity declines during physiological ageing in stromal cells, and mimicking such decline through genetic inactivation of YAP/TAZ in these cells leads to accelerated ageing. Conversely, sustaining YAP function rejuvenates old cells and opposes the emergence of ageing-related traits associated with either physiological ageing or accelerated ageing triggered by a mechano-defective extracellular matrix. Ageing traits induced by inactivation of YAP/TAZ are preceded by induction of tissue senescence. This occurs because YAP/TAZ mechanotransduction suppresses cGAS-STING signalling, to the extent that inhibition of STING prevents tissue senescence and premature ageing-related tissue degeneration after YAP/TAZ inactivation. Mechanistically, YAP/TAZ-mediated control of cGAS-STING signalling relies on the unexpected role of YAP/TAZ in preserving nuclear envelope integrity, at least in part through direct transcriptional regulation of lamin B1 and ACTR2, the latter of which is involved in building the peri-nuclear actin cap. The findings demonstrate that declining YAP/TAZ mechanotransduction drives ageing by unleashing cGAS-STING signalling, a pillar of innate immunity. Thus, sustaining YAP/TAZ mechanosignalling or inhibiting STING may represent promising approaches for limiting senescence-associated inflammation and improving healthy ageing.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures









Comment in
-
YAP/TAZ get a STING in the tail.Nat Rev Mol Cell Biol. 2022 Sep;23(9):581. doi: 10.1038/s41580-022-00519-1. Nat Rev Mol Cell Biol. 2022. PMID: 35831513 No abstract available.
-
YAP/TAZ dull the STING of aging.J Cardiovasc Aging. 2022;2:44. doi: 10.20517/jca.2022.33. Epub 2022 Aug 5. J Cardiovasc Aging. 2022. PMID: 36337727 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

