Epidemiologically-based strategies for the detection of emerging plant pathogens
- PMID: 35768558
- PMCID: PMC9243127
- DOI: 10.1038/s41598-022-13553-y
Epidemiologically-based strategies for the detection of emerging plant pathogens
Abstract
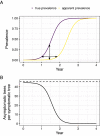
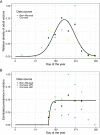
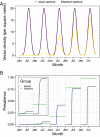
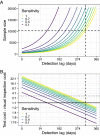
Emerging pests and pathogens of plants are a major threat to natural and managed ecosystems worldwide. Whilst it is well accepted that surveillance activities are key to both the early detection of new incursions and the ability to identify pest-free areas, the performance of these activities must be evaluated to ensure they are fit for purpose. This requires consideration of the number of potential hosts inspected or tested as well as the epidemiology of the pathogen and the detection method used. In the case of plant pathogens, one particular concern is whether the visual inspection of plant hosts for signs of disease is able to detect the presence of these pathogens at low prevalences, given that it takes time for these symptoms to develop. One such pathogen is the ST53 strain of the vector-borne bacterial pathogen Xylella fastidiosa in olive hosts, which was first identified in southern Italy in 2013. Additionally, X. fastidiosa ST53 in olive has a rapid rate of spread, which could also have important implications for surveillance. In the current study, we evaluate how well visual surveillance would be expected to perform for this pathogen and investigate whether molecular testing of either tree hosts or insect vectors offer feasible alternatives. Our results identify the main constraints to each of these strategies and can be used to inform and improve both current and future surveillance activities.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Xylella fastidiosa: Insights into an Emerging Plant Pathogen.Annu Rev Phytopathol. 2018 Aug 25;56:181-202. doi: 10.1146/annurev-phyto-080417-045849. Epub 2018 Jun 11. Annu Rev Phytopathol. 2018. PMID: 29889627 Review.
-
Network analysis reveals why Xylella fastidiosa will persist in Europe.Sci Rep. 2017 Mar 6;7(1):71. doi: 10.1038/s41598-017-00077-z. Sci Rep. 2017. PMID: 28250430 Free PMC article.
-
Xylella fastidiosa subsp. pauca and olive produced lipids moderate the switch adhesive versus non-adhesive state and viceversa.PLoS One. 2020 May 15;15(5):e0233013. doi: 10.1371/journal.pone.0233013. eCollection 2020. PLoS One. 2020. PMID: 32413086 Free PMC article.
-
Xylella fastidiosa, a new plant pathogen that threatens global farming: Ecology, molecular biology, search for remedies.Biochem Biophys Res Commun. 2018 Jul 12;502(2):173-182. doi: 10.1016/j.bbrc.2018.05.073. Biochem Biophys Res Commun. 2018. PMID: 29887124
-
Aphrophoridae Role in Xylella fastidiosa subsp. pauca ST53 Invasion in Southern Italy.Pathogens. 2021 Aug 16;10(8):1035. doi: 10.3390/pathogens10081035. Pathogens. 2021. PMID: 34451499 Free PMC article. Review.
Cited by
-
Translating virome analyses to support biosecurity, on-farm management, and crop breeding.Front Plant Sci. 2023 Mar 14;14:1056603. doi: 10.3389/fpls.2023.1056603. eCollection 2023. Front Plant Sci. 2023. PMID: 36998684 Free PMC article. Review.
-
Using 'sentinel' plants to improve early detection of invasive plant pathogens.PLoS Comput Biol. 2023 Feb 2;19(2):e1010884. doi: 10.1371/journal.pcbi.1010884. eCollection 2023 Feb. PLoS Comput Biol. 2023. PMID: 36730434 Free PMC article.
-
Assessing delimiting strategies to identify the infested zones of quarantine plant pests and diseases.Sci Rep. 2025 Feb 15;15(1):5610. doi: 10.1038/s41598-025-90343-2. Sci Rep. 2025. PMID: 39955457 Free PMC article.
References
-
- Brasier CM. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008;57:792–808. doi: 10.1111/j.1365-3059.2008.01886.x. - DOI
-
- IPPC. Surveillance guide—A guide to understand the principal requirements of surveillance programmes for national plant protection organizations. Second edition.http://www.fao.org/documents/card/en/c/cb7139en (2021) 10.4060/cb7139en.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

