Target deconvolution studies of (2R,6R)-hydroxynorketamine: an elusive search
- PMID: 35768639
- PMCID: PMC10013843
- DOI: 10.1038/s41380-022-01673-w
Target deconvolution studies of (2R,6R)-hydroxynorketamine: an elusive search
Abstract
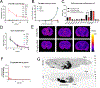
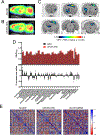
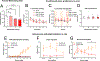
The off-label use of racemic ketamine and the FDA approval of (S)-ketamine are promising developments for the treatment of depression. Nevertheless, racemic ketamine and (S)-ketamine are controlled substances with known abuse potential and their use is associated with undesirable side effects. For these reasons, research efforts have focused on identifying alternatives. One candidate is (2R,6R)-hydroxynorketamine ((2R,6R)-HNK), a ketamine metabolite that in preclinical models lacks the dissociative and abuse properties of ketamine while retaining its antidepressant-like behavioral efficacy. (2R,6R)-HNK's mechanism of action however is unclear. The main goals of this study were to perform an in-depth pharmacological characterization of (2R,6R)-HNK at known ketamine targets, to use target deconvolution approaches to discover novel proteins that bind to (2R,6R)-HNK, and to characterize the biodistribution and behavioral effects of (2R,6R)-HNK across several procedures related to substance use disorder liability. We found that unlike (S)- or (R)-ketamine, (2R,6R)-HNK did not directly bind to any known or proposed ketamine targets. Extensive screening and target deconvolution experiments at thousands of human proteins did not identify any other direct (2R,6R)-HNK-protein interactions. Biodistribution studies using radiolabeled (2R,6R)-HNK revealed non-selective brain regional enrichment, and no specific binding in any organ other than the liver. (2R,6R)-HNK was inactive in conditioned place preference, open-field locomotor activity, and intravenous self-administration procedures. Despite these negative findings, (2R,6R)-HNK produced a reduction in immobility time in the forced swim test and a small but significant increase in metabolic activity across a network of brain regions, and this metabolic signature differed from the brain metabolic profile induced by ketamine enantiomers. In sum, our results indicate that (2R,6R)-HNK does not share pharmacological or behavioral profile similarities with ketamine or its enantiomers. However, it could still be possible that both ketamine and (2R,6R)-HNK exert antidepressant-like efficacy through a common and previously unidentified mechanism. Given its pharmacological profile, we predict that (2R,6R)-HNK will exhibit a favorable safety profile in clinical trials, and we must wait for clinical studies to determine its antidepressant efficacy.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Conflict of interest.
CZ is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation. CZ and RM are co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydroxylated and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. PZ, RM, PM, CJT, CAZ and TDG are co-inventors on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders, and on a patent on the crystal forms and methods of synthesis of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine. PM and CJT are co-inventors on a patent application for the salts of (2R,6R)-hydroxynorketamine, their crystal forms, and methods of making the same and the process for synthesis and purification of (2R,6R)-hydroxynorketamine. RM, PM, CAZ, and CT have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. PZ and TDG have assigned their patent rights to the University of Maryland Baltimore but will share a percentage of any royalties that may be received by the University of Maryland Baltimore. MM has received research funding from AstraZeneca, Redpin Therapeutics and Attune Neuroscience. All other authors declare no conflicts of interest.
Figures
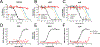

Comment in
-
Comments to behavioral tests for antidepressant-like actions of (2R,6R)-hydroxynorketamine by Bonaventura et al.Mol Psychiatry. 2024 Jan;29(1):3-4. doi: 10.1038/s41380-022-01766-6. Epub 2022 Sep 13. Mol Psychiatry. 2024. PMID: 36100667 No abstract available.
References
-
- Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176:422–424. - PubMed
-
- Schatzberg AF. A word to the wise about ketamine. Am J Psychiatry. 2014;171:262–264. - PubMed
-
- Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008;21:14–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

