Meaningful Improvement in General Health Outcomes with Guselkumab Treatment for Psoriatic Arthritis: Patient-Reported Outcomes Measurement Information System-29 Results from a Phase 3 Study
- PMID: 35768650
- PMCID: PMC9584870
- DOI: 10.1007/s40271-022-00588-6
Meaningful Improvement in General Health Outcomes with Guselkumab Treatment for Psoriatic Arthritis: Patient-Reported Outcomes Measurement Information System-29 Results from a Phase 3 Study
Abstract
Objective: The Phase 3 DISCOVER-1 study of guselkumab is the first randomized controlled trial to use Patient-Reported Outcomes Measurement Information System (PROMIS) measures to assess the effects of treatment on general health outcomes in patients with psoriatic arthritis (PsA).
Methods: Patients (N = 381) with active PsA were randomized 1:1:1 to guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at Week 0, Week 4, then every 8 weeks (Q8W); or placebo with Week 24 crossover to guselkumab Q4W. The PROMIS-29 Profile contains four items for each of seven domains (anxiety, depression, fatigue, pain interference, physical function, sleep disturbance, and social participation) and one pain-intensity item. Raw domain scores are converted to standardized T-scores, with norms based on a US general population mean of 50 (1 standard deviation (SD) = 10). T-score changes of ≥ 5 are considered clinically meaningful. Least-squares mean PROMIS-29 T-score changes from baseline to Week 24 and Week 52 were summarized for the guselkumab and placebo groups; nominal p-values comparing results between guselkumab and placebo were calculated at Week 24 using a mixed model for repeated measures. The proportions of patients who achieved clinically meaningful improvement in PROMIS-29 T-scores were also summarized at Week 24 and Week 52; nominal p-values comparing results between guselkumab and placebo were calculated at Week 24 using the Cochran-Mantel-Haenszel test.
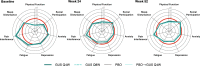
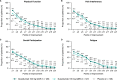
Results: In the DISCOVER-1 patient population, mean PROMIS-29 T-scores at baseline were ~ 1 SD worse for physical function and pain interference and were numerically worse for social participation, fatigue, and sleep disturbance compared with the US general population. At Week 24, mean PROMIS-29 T-scores improved in guselkumab-treated patients, approaching US population norms; T-scores continued to improve through Week 52. Significantly higher proportions of patients in both guselkumab treatment arms (31-52% across domains) had clinically meaningful improvements in pain interference, fatigue, physical function, sleep, and social participation at Week 24 versus placebo (all nominal p ≤ 0.05).
Conclusion: In patients with active PsA, guselkumab treatment provided clinically meaningful reductions in fatigue and pain and improvement in physical function and social participation, as measured by the PROMIS-29 Profile. These improvements were maintained through 1 year.
Clinicaltrials: GOV: Registration number, NCT03162796; Submission date 19 May 2017.
© 2022. The Author(s).
Conflict of interest statement
Ana-Maria Orbai has received grant/research support from AbbVie, Amgen, Eli Lilly and Company, Celgene, Novartis, Janssen, and Horizon; has received consulting fees from Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, and is also a private consultant or advisor for Sun Pharmaceutical Industries, Inc, not in her capacity as a Johns Hopkins faculty member and was not compensated for this service. Laura C. Coates has received grant/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; speaker fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Medac, Novartis, Pfizer, and UCB; and consultant fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, and UCB. Laura C Coates is funded by a National Institute for Health Research (NIHR) Clinician Scientist award. The research was supported by the NIHR Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. We acknowledge the support of the NIHR Clinical Research Network. Atul Deodhar has received consulting fees for participation in Advisory Boards from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; grant/research support from AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB; and speaker fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Philip S. Helliwell has received consulting fees from Eli Lilly and fees for educational services from AbbVie, Amgen, Novartis, and Janssen. Christopher T. Ritchlin has received grant/research support from UCB Pharma, AbbVie, and Amgen; consulting fees from UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, and Janssen. Evan Leibowitz is an employee of Janssen Scientific Affairs, LLC, and owns stock in Johnson & Johnson, of which Janssen Scientific Affairs is a wholly owned subsidiary. Alexa P. Kollmeier, Elizabeth C. Hsia, Xie L. Xu, Shihong Sheng, Yan Liu, and Chenglong Han are employees of Janssen Research & Development, LLC, and own stock in Johnson & Johnson, of which Janssen Research & Development is a wholly owned subsidiary. Yusang Jiang is a consultant employed by Cytel, Inc. and funded by Janssen to provide statistical support.
Figures
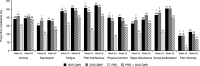

Comment in
-
Concerns about the Responsiveness of Generic Measures and the Search for a "Minimally" Important Change: Today's PRO Red Herrings.Patient. 2022 Nov;15(6):655-656. doi: 10.1007/s40271-022-00590-y. Epub 2022 Jul 2. Patient. 2022. PMID: 35779179 No abstract available.
Similar articles
-
Guselkumab demonstrated an independent treatment effect in reducing fatigue after adjustment for clinical response-results from two phase 3 clinical trials of 1120 patients with active psoriatic arthritis.Arthritis Res Ther. 2021 Jul 14;23(1):190. doi: 10.1186/s13075-021-02554-3. Arthritis Res Ther. 2021. PMID: 34261541 Free PMC article. Clinical Trial.
-
The Effect of Guselkumab on General Health State in Biologic-Naïve Patients with Active Psoriatic Arthritis Through Week 52 of the Phase 3, Randomized, Placebo-Controlled DISCOVER-2 Trial.Adv Ther. 2022 Oct;39(10):4632-4644. doi: 10.1007/s12325-022-02269-0. Epub 2022 Aug 10. Adv Ther. 2022. PMID: 35947348 Clinical Trial.
-
The Effect of Guselkumab on Work Productivity in Biologic-Naïve Patients with Active Psoriatic Arthritis Through Week 52 of the Phase 3, Randomized, Placebo-Controlled DISCOVER-2 Trial.Adv Ther. 2022 Oct;39(10):4613-4631. doi: 10.1007/s12325-022-02270-7. Epub 2022 Aug 10. Adv Ther. 2022. PMID: 35947349 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Does Sharing Patient-Reported Outcomes with Doctors Improve Patient Symptoms? [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2018 May. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2018 May. PMID: 37099662 Free Books & Documents. Review.
Cited by
-
Sleep Problems in Patients With Psoriatic Arthritis: A Systematic Literature Review and Metaanalysis.J Rheumatol. 2023 May 1:jrheum.2022-1169. doi: 10.3899/jrheum.2022-1169. Online ahead of print. J Rheumatol. 2023. PMID: 37127321 Free PMC article.
References
-
- Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

