White matter hyperintensities are a prominent feature of autosomal dominant Alzheimer's disease that emerge prior to dementia
- PMID: 35768838
- PMCID: PMC9245224
- DOI: 10.1186/s13195-022-01030-7
White matter hyperintensities are a prominent feature of autosomal dominant Alzheimer's disease that emerge prior to dementia
Abstract
Background: To promote the development of effective therapies, there is an important need to characterize the full spectrum of neuropathological changes associated with Alzheimer's disease. In line with this need, this study examined white matter abnormalities in individuals with early-onset autosomal dominant Alzheimer's disease, in relation to age and symptom severity.
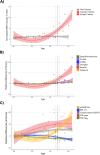
Methods: This is a cross-sectional analysis of data collected in members of a large kindred with a PSEN1 E280A mutation. Participants were recruited between September 2011 and July 2012 from the Colombian Alzheimer's Prevention Initiative registry. The studied cohort comprised 50 participants aged between 20 and 55 years, including 20 cognitively unimpaired mutation carriers, 9 cognitively impaired mutation carriers, and 21 non-carriers. Participants completed an MRI, a lumbar puncture for cerebrospinal fluid collection, a florbetapir PET scan, and neurological and neuropsychological examinations. The volume of white matter hyperintensities (WMH) was compared between cognitively unimpaired carriers, cognitively impaired carriers, and non-carriers. Relationships between WMH, age, and cognitive performance were further examined in mutation carriers.
Results: The mean (SD) age of participants was 35.8 (9.6) years and 64% were women. Cardiovascular risk factors were uncommon and did not differ across groups. Cognitively impaired carriers [median, 6.37; interquartile range (IQR), 9.15] had an increased volume of WMH compared to both cognitively unimpaired carriers [median, 0.85; IQR, 0.79] and non-carriers [median, 1.07; IQR, 0.71]. In mutation carriers, the volume of WMH strongly correlated with cognition and age, with evidence for an accelerated rate of changes after the age of 43 years, 1 year earlier than the estimated median age of symptom onset. In multivariable regression models including cortical amyloid retention, superior parietal lobe cortical thickness, and cerebrospinal fluid phospho-tau, the volume of WMH was the only biomarker independently and significantly contributing to the total explained variance in cognitive performance.
Conclusions: The volume of WMH is increased among individuals with symptomatic autosomal-dominant Alzheimer's disease, begins to increase prior to clinical symptom onset, and is an independent determinant of cognitive performance in this group. These findings suggest that WMH are a key component of autosomal-dominant Alzheimer's disease that is closely related to the progression of clinical symptoms.
Keywords: Autosomal-dominant Alzheimer’s disease; Cerebral microbleeds; Cognition; Dementia; PSEN1; White matter hyperintensities.
© 2022. The Author(s).
Conflict of interest statement
FL reports funding for the Colombian API Registry from Genentech. YTQ reports consulting fees from Biogen. The other authors declare that they have no competing interests.
Figures

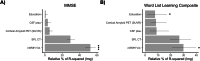
References
-
- Bangen KJ, Preis SR, Delano-Wood L, Wolf PA, Libon DJ, Bondi MW, et al. Baseline white matter hyperintensities and hippocampal volume are associated with conversion from normal cognition to mild cognitive impairment in the Framingham Offspring Study. Alzheimer Dis Assoc Disord. 2018;32(1):50. doi: 10.1097/WAD.0000000000000215. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

