Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells-an in-vitro study
- PMID: 35768853
- PMCID: PMC9245296
- DOI: 10.1186/s13005-022-00322-5
Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells-an in-vitro study
Erratum in
-
Correction: Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells-an in‑vitro study.Head Face Med. 2023 Aug 18;19(1):35. doi: 10.1186/s13005-023-00386-x. Head Face Med. 2023. PMID: 37596585 Free PMC article. No abstract available.
Abstract
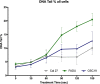
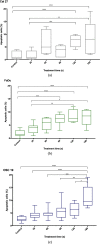
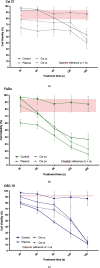
Background: The aim of the present study was to examine the cytostatic effects of cold atmospheric plasma (CAP) on different head and neck squamous carcinoma (HNSCC) cell lines either in isolation or in combination with low dose cisplatin. The effect of CAP treatment was investigated by using three different HNSCC cell lines (chemo-resistant Cal 27, chemo-sensitive FaDu and OSC 19).
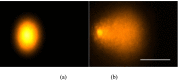
Materials and method: Cell lines were exposed to CAP treatment for 30, 60, 90, 120 and 180 s (s). Cisplatin was added concurrently (cc) or 24 h after CAP application (cs). Cell viability, DNA damage and apoptosis was evaluated by dye exclusion, MTT, alkaline microgel electrophoresis assay and Annexin V-Fit-C/PI respectively.
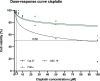
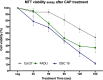
Results: In all cell lines, 120 s of CAP exposure resulted in a significant reduction of cell viability. DNA damage significantly increased after 60 s. Combined treatment of cells with CAP and low dose cisplatin showed additive effects. A possible sensitivity to cisplatin could be restored in Cal 27 cells by CAP application.
Conclusion: CAP shows strong cytostatic effects in HNSCC cell lines that can be increased by concurrent cisplatin treatment, suggesting that CAP may enhance the therapeutic efficacy of low dose cisplatin.
Keywords: Adjuvant combination therapy; Cold atmospheric plasma; Head and neck cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. 10.1016/j.ejca.2012.12.027. - PubMed
-
- Cooper JS, et al. Precisely defining high-risk operable head and neck tumors based on rtog #85-03 and #88-24: Targets for postoperative radiochemotherapy? Head Neck. 1999;20(7):588–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

