Early versus delayed treatment with glatiramer acetate: Analysis of up to 27 years of continuous follow-up in a US open-label extension study
- PMID: 35768939
- PMCID: PMC9442630
- DOI: 10.1177/13524585221094239
Early versus delayed treatment with glatiramer acetate: Analysis of up to 27 years of continuous follow-up in a US open-label extension study
Abstract
Background: Glatiramer acetate (GA) is US-approved for relapsing multiple sclerosis.
Objectives: To describe GA long-term clinical profile. To compare effectiveness of early start (ES) versus delayed start (DS; up to 3 years) with GA.
Methods: Phase 3 trial participants entered a randomized placebo-controlled period then an open-label extension (OLE) with GA.
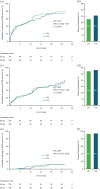
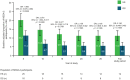
Results: Overall, 208 out of 251 (82.9%) randomized participants entered the OLE; 24 out of 101 (23.8%, ES) and 28 out of 107 (26.2%, DS) participants completed the OLE. Median GA treatment was 9.8 (0.1-26.3) years. Annualized change in Expanded Disability Status Scale (EDSS) score was lower with ES versus DS (p = 0.0858: full study; p = 0.002; Year 5). Participants with improved/stable EDSS was consistently higher with ES versus DS: 40.3% versus 31.6% (p = 0.1590; full study); 70.8% versus 55.6% (p = 0.015; Year 5). ES prolonged time-to-6-month confirmed disease worsening (CDW) versus DS (9.8 vs 6.7 years), time-to-12-month CDW (18.9 vs 11.6 years), and significantly reduced time-to-second-6-month CDW (p = 0.0441). No new safety concerns arose.
Conclusion: GA long-term treatment maintained clinical benefit with a similar safety profile to phase 3 results; a key limitation was that only 25% of participants completed the OLE. Early initiation of GA had sustained benefits versus delayed treatment.
Keywords: Clinical trial; disease-modifying therapies; glatiramer acetate; multiple sclerosis; quality of life; relapsing/remitting.
Conflict of interest statement
Figures





References
-
- Thompson AJ, Baranzini SE, Geurts J, et al.. Multiple sclerosis. Lancet 2018; 391(10130): 1622–1636. - PubMed
-
- Freedman MS, Devonshire V, Duquette P, et al.. Treatment optimization in multiple sclerosis: Canadian MS Working Group Recommendations. Can J Neurol Sci 2020; 47(4): 437–455. - PubMed
-
- Grand’Maison F, Yeung M, Morrow SA, et al.. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: A theoretical approach to optimizing treatment. Curr Med Res Opin 2018; 34(8): 1419–1430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

