Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn's disease?
- PMID: 35768996
- PMCID: PMC9540440
- DOI: 10.1111/apt.17089
Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn's disease?
Abstract
Background: During illness, adaptations of the hypothalamic-pituitary-thyroid axis reduce energy expenditure, protein catabolism and modulate immune responses to promote survival. Lower serum free triiodothyronine-to-thyroxine (fT3/fT4) ratio has been linked to non-response to treatment in a range of diseases, including in biologic-treated patients with inflammatory bowel disease.
Aim: To assess whether baseline serum fT3/fT4 ratio predicted primary non-response (PNR) and non-remission to infliximab and adalimumab in patients with Crohn's disease METHODS: Thyroid function tests were undertaken in stored serum from biologic-naïve adult patients with active luminal Crohn's disease immediately prior to treatment with infliximab (427 originator; 122 biosimilar) or adalimumab (448) in the Personalised Anti-TNF Therapy in Crohn's Disease study (PANTS).
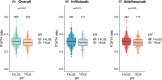
Results: Baseline median [IQR] fT3/fT4 ratios were lower in women than men (0.30 [0.27-0.34] vs 0.32 [0.28-0.36], p < 0.001), in patients with more severe inflammatory disease, and in patients receiving corticosteroids (0.28 [0.25-0.33] vs. 0.32 [0.29-0.36], p < 0.001). Multivariable logistic regression analysis demonstrated that fT3/fT4 ratio was independently associated with PNR at week 14 (odds ratio [OR] 0.51, 95% confidence interval [CI] 0.31-0.85, p = 0.009), but not non-remission or changes in faecal calprotectin concentrations at week 54. The optimal threshold to determine PNR was 0.31 (area under the curve 0.57 [95% CI 0.54-0.61], sensitivity 0.62 [95% CI 0.41-0.74], and specificity 0.53 [95% CI 0.42-0.73]).
Conclusions: Lower baseline serum fT3/fT4 ratio was associated with female sex, corticosteroid use and disease activity. It predicted PNR to anti-TNF treatment at week 14, but not non-remission at week 54.
Keywords: Crohn’s disease; IBD; PANTS; T3; T4; TSH; low T3 syndrome; low T3/T4 ratio; non-thyroidal illness syndrome; sick euthyroid syndrome.
© 2022 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Lin reports non‐financial support from Pfizer, non‐financial support from Ferring, outside the submitted work. Dr. Kennedy reports grants from F. Hoffmann‐La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non‐financial support from Immundiagnostik, grants and non‐financial support from AbbVie, grants and personal fees from Celltrion, personal fees and non‐financial support from Janssen, personal fees from Takeda, personal fees and non‐financial support from Dr Falk, outside the submitted work.
Prof. Ahmad reports grants and non‐financial support from F. Hoffmann‐La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non‐financial support from Immundiagnostik, personal fees from Biogen inc, grants and personal fees from Celltrion Healthcare, personal fees and non‐financial support from Immundiagnostik, personal fees from Takeda, personal fees from ARENA, personal fees from Gilead, personal fees from Adcock Ingram Healthcare, personal fees from Pfizer, personal fees from Genentech, non‐financial support from Tillotts, outside the submitted work. Dr Selinger has received unrestricted research grants from Warner Chilcott, Janssen and AbbVie, has provided consultancy to Warner Chilcott, Dr Falk, AbbVie, Takeda, Fresenius Kabi, Arena, Galapagos, Celltrion, Norgine and Janssen, and had speaker arrangements with Warner Chilcott, Norgine, Galapagos, Fresenius Kabi, Celtrion, Dr Falk, AbbVie, MSD, Pfizer and Takeda. Dr. Goodhand reports grants from F. Hoffmann‐La Roche AG, grants from Biogen Inc, grants from Celltrion Healthcare, grants from Galapagos NV, non‐financial support from Immundiagnostik, outside the submitted work. The following authors have nothing to declare: Neil Chanchlani, Isabel Carbery, Malik Janjua, Rachel Nice, Timothy J McDonald and Claire Bewshea.
Figures




Comment in
-
Editorial: serum triiodothyronine-to-thyroxine (T3/T4) ratio as biomarker of response in IBD - an open issue.Aliment Pharmacol Ther. 2022 Sep;56(5):903-904. doi: 10.1111/apt.17136. Aliment Pharmacol Ther. 2022. PMID: 35934852 No abstract available.
Similar articles
-
Pretreatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients With Active Luminal Crohn's Disease.Crohns Colitis 360. 2023 May 15;5(3):otad026. doi: 10.1093/crocol/otad026. eCollection 2023 Jul. Crohns Colitis 360. 2023. PMID: 37265586 Free PMC article.
-
Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study.Lancet Gastroenterol Hepatol. 2019 May;4(5):341-353. doi: 10.1016/S2468-1253(19)30012-3. Epub 2019 Feb 27. Lancet Gastroenterol Hepatol. 2019. PMID: 30824404
-
Mechanisms and management of loss of response to anti-TNF therapy for patients with Crohn's disease: 3-year data from the prospective, multicentre PANTS cohort study.Lancet Gastroenterol Hepatol. 2024 Jun;9(6):521-538. doi: 10.1016/S2468-1253(24)00044-X. Epub 2024 Apr 16. Lancet Gastroenterol Hepatol. 2024. PMID: 38640937
-
[Anti-TNF therapy in treatment of luminal Crohn's disease].Acta Med Croatica. 2013 Apr;67(2):179-89. Acta Med Croatica. 2013. PMID: 24471301 Review. Croatian.
-
Visceral adiposity and inflammatory bowel disease.Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review.
Cited by
-
Pretreatment Vitamin D Concentrations Do Not Predict Therapeutic Outcome to Anti-TNF Therapies in Biologic-Naïve Patients With Active Luminal Crohn's Disease.Crohns Colitis 360. 2023 May 15;5(3):otad026. doi: 10.1093/crocol/otad026. eCollection 2023 Jul. Crohns Colitis 360. 2023. PMID: 37265586 Free PMC article.
-
Precision medicine in inflammatory bowel disease.Precis Clin Med. 2023 Dec 18;6(4):pbad033. doi: 10.1093/pcmedi/pbad033. eCollection 2023 Dec. Precis Clin Med. 2023. PMID: 38638127 Free PMC article. Review.
-
Mechanism and potential treatments for gastrointestinal dysfunction in patients with COVID-19.World J Gastroenterol. 2022 Dec 28;28(48):6811-6826. doi: 10.3748/wjg.v28.i48.6811. World J Gastroenterol. 2022. PMID: 36632313 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
