Monoallelic and biallelic mutations in RELN underlie a graded series of neurodevelopmental disorders
- PMID: 35769015
- PMCID: PMC9989350
- DOI: 10.1093/brain/awac164
Monoallelic and biallelic mutations in RELN underlie a graded series of neurodevelopmental disorders
Abstract
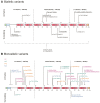
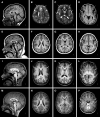
Reelin, a large extracellular protein, plays several critical roles in brain development and function. It is encoded by RELN, first identified as the gene disrupted in the reeler mouse, a classic neurological mutant exhibiting ataxia, tremors and a 'reeling' gait. In humans, biallelic variants in RELN have been associated with a recessive lissencephaly variant with cerebellar hypoplasia, which matches well with the homozygous mouse mutant that has abnormal cortical structure, small hippocampi and severe cerebellar hypoplasia. Despite the large size of the gene, only 11 individuals with RELN-related lissencephaly with cerebellar hypoplasia from six families have previously been reported. Heterozygous carriers in these families were briefly reported as unaffected, although putative loss-of-function variants are practically absent in the population (probability of loss of function intolerance = 1). Here we present data on seven individuals from four families with biallelic and 13 individuals from seven families with monoallelic (heterozygous) variants of RELN and frontotemporal or temporal-predominant lissencephaly variant. Some individuals with monoallelic variants have moderate frontotemporal lissencephaly, but with normal cerebellar structure and intellectual disability with severe behavioural dysfunction. However, one adult had abnormal MRI with normal intelligence and neurological profile. Thorough literature analysis supports a causal role for monoallelic RELN variants in four seemingly distinct phenotypes including frontotemporal lissencephaly, epilepsy, autism and probably schizophrenia. Notably, we observed a significantly higher proportion of loss-of-function variants in the biallelic compared to the monoallelic cohort, where the variant spectrum included missense and splice-site variants. We assessed the impact of two canonical splice-site variants observed as biallelic or monoallelic variants in individuals with moderately affected or normal cerebellum and demonstrated exon skipping causing in-frame loss of 46 or 52 amino acids in the central RELN domain. Previously reported functional studies demonstrated severe reduction in overall RELN secretion caused by heterozygous missense variants p.Cys539Arg and p.Arg3207Cys associated with lissencephaly suggesting a dominant-negative effect. We conclude that biallelic variants resulting in complete absence of RELN expression are associated with a consistent and severe phenotype that includes cerebellar hypoplasia. However, reduced expression of RELN remains sufficient to maintain nearly normal cerebellar structure. Monoallelic variants are associated with incomplete penetrance and variable expressivity even within the same family and may have dominant-negative effects. Reduced RELN secretion in heterozygous individuals affects only cortical structure whereas the cerebellum remains intact. Our data expand the spectrum of RELN-related neurodevelopmental disorders ranging from lethal brain malformations to adult phenotypes with normal brain imaging.
Keywords: RELN; Reelin; autism; epilepsy; lissencephaly.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- D'Arcangelo G, Curran T. Reeler: New tales on an old mutant mouse. Bioessays. 1998;20:235–244. - PubMed
-
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. - PubMed
-
- Falconer DS. Two new mutations, trembler and reeler, with neurological actions in the house mouse (Mus musculus l). J Genet. 1951;50:192–201. - PubMed
-
- Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–96. - PubMed
-
- Hong SE, Shugart YY, Huang DT, et al. Correction: Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2001;27:225–225. - PubMed

