Pharmacokinetics, safety, and tolerability of gepotidacin administered as single or repeat ascending doses, in healthy adults and elderly subjects
- PMID: 35769034
- PMCID: PMC9468557
- DOI: 10.1111/cts.13359
Pharmacokinetics, safety, and tolerability of gepotidacin administered as single or repeat ascending doses, in healthy adults and elderly subjects
Abstract
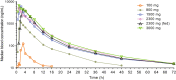
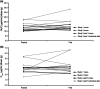
Gepotidacin, a novel, first-in-class triazaacenaphthylene antibiotic, inhibits bacterial DNA replication by a distinct mechanism of action. We report the pharmacokinetics (PKs), safety, and tolerability of gepotidacin following single or multiple ascending doses. Studies 1 and 2 were randomized, single-blind, placebo-controlled trials in healthy adults aged 18-60 years, who received single (study 1 [NCT02202187]; 100-3000 mg) or repeat (study 2 [NCT01706315]; 400 mg twice daily to 2000 mg thrice daily) ascending doses of gepotidacin. Study 3 (NCT02045849) was an open-label, three-part, study in healthy adults; here, we report on part 3, a two-period, repeat-dose, crossover study. Healthy elderly participants received repeat 1500 mg gepotidacin twice daily with or without a moderate-fat meal. Primary end points were PKs (studies 1 and 2) and safety (studies 1 and 3 part 3). Gepotidacin PK parameters were comparable across all ages and were dose proportional. In all studies, gepotidacin was readily absorbed with median time to maximum concentration observed ranging from 1.0 to 4.0 h across all doses. Median apparent terminal phase half-life was consistent across studies and doses (range: 5.97-19.2 h). Steady-state was achieved following repeated dosing for 3-5 days; gepotidacin PK parameters were time invariant after repeated oral dosing. A moderate-fat meal did not affect gepotidacin PK parameters. Gepotidacin was generally well-tolerated, with no drug-related serious adverse events reported. Collectively, these PK and safety data across a wide range of doses in healthy participants aged greater than or equal to 18 years support the development of gepotidacin in further clinical studies.
© 2022 GSK. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
C.T., E.F.D. and M.H. are former employees of and past/current shareholders in GSK. B.S. and M.S. are employees of and shareholders in GSK.
Figures

References
-
- Mushtaq S, Vickers A, Sadouki Z, et al. In‐vitro activities of gepotidacin, a novel triazaacenaphthylene and topoisomerase IV DNA gyrase inhibitor, against gram‐negative bacteria and staphylococcus saprophyticus. ECCMID. 2019. abstract P1849.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

