Perspectives for the Use of Bacterial Lysates for the Treatment of Allergic Rhinitis: A Systematic Review
- PMID: 35769192
- PMCID: PMC9236485
- DOI: 10.2147/JAA.S360828
Perspectives for the Use of Bacterial Lysates for the Treatment of Allergic Rhinitis: A Systematic Review
Abstract
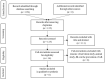
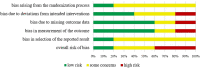
Bacterial lysates (BLs) are mixtures of bacterial antigens that have been used for many decades to minimize the risk of recurrent respiratory tract infections in both pediatric and adult populations. Research on the use of BLs is also conducted in allergology. Biomedical databases were searched for articles on the use of BLs in the treatment of allergic rhinitis (AR). After rejecting ineligible articles, six remaining reports were reviewed. Based on this review, it can be concluded that adding BL to standard therapy for seasonal or perennial AR reduces the severity of nasal symptoms and the need for antiallergic medications in both children and adults. Concurrently, these formulations have a high safety profile. An analysis of studies shows that the first effects of BLs therapy appear at the earliest 2-6 weeks after the start of treatment and persist at least 3 months after treatment.
Keywords: allergic rhinitis; bacterial lysate; immunostimulation; treatment.
© 2022 Janeczek et al.
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article. No funding was received to assist with the preparation of this manuscript.
Figures
References
-
- Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi:10.1016/S0140-6736(06)69283-0 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials