Telomere Attrition in Induced Pluripotent Stem Cell-Derived Neurons From ALS/FTD-Related C9ORF72 Repeat Expansion Carriers
- PMID: 35769259
- PMCID: PMC9234284
- DOI: 10.3389/fcell.2022.874323
Telomere Attrition in Induced Pluripotent Stem Cell-Derived Neurons From ALS/FTD-Related C9ORF72 Repeat Expansion Carriers
Abstract
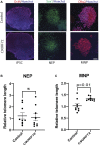
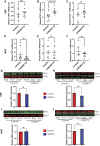
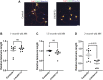
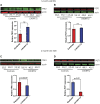
The GGGGCC (G4C2) repeat expansion in C9ORF72 is the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Dysregulated DNA damage response and the generation of reactive oxygen species (ROS) have been postulated as major drivers of toxicity in C9ORF72 pathogenesis. Telomeres are tandem-repeated nucleotide sequences that are located at the end of chromosomes and protect them from degradation. Interestingly, it has been established that telomeres are sensitive to ROS. Here, we analyzed telomere length in neurons and neural progenitor cells from several induced pluripotent stem cell (iPSC) lines from control subjects and C9ORF72 repeat expansion carriers. We found an age-dependent decrease in telomere length in two-month-old iPSC-derived motor neurons from C9ORF72 carriers as compared to control subjects and a dysregulation in the protein levels of shelterin complex members TRF2 and POT1.
Keywords: Amyotrophic lateral sclerosis, Frontotemporal dementia; C9orf72; induced pluripotent stem cells; motor neuron differentiation; telomeres.
Copyright © 2022 Robinson, Ali, Diaz-Hernandez and Lopez-Gonzalez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as potential conflicts of interest.
Figures

Similar articles
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
-
Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons.Acta Neuropathol. 2013 Sep;126(3):385-99. doi: 10.1007/s00401-013-1149-y. Epub 2013 Jul 9. Acta Neuropathol. 2013. PMID: 23836290 Free PMC article.
-
ADAR2 mislocalization and widespread RNA editing aberrations in C9orf72-mediated ALS/FTD.Acta Neuropathol. 2019 Jul;138(1):49-65. doi: 10.1007/s00401-019-01999-w. Epub 2019 Apr 3. Acta Neuropathol. 2019. PMID: 30945056 Free PMC article.
-
Poly(GR) in C9ORF72-Related ALS/FTD Compromises Mitochondrial Function and Increases Oxidative Stress and DNA Damage in iPSC-Derived Motor Neurons.Neuron. 2016 Oct 19;92(2):383-391. doi: 10.1016/j.neuron.2016.09.015. Epub 2016 Oct 6. Neuron. 2016. PMID: 27720481 Free PMC article.
-
Role of the C9ORF72 Gene in the Pathogenesis of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia.Neurosci Bull. 2020 Sep;36(9):1057-1070. doi: 10.1007/s12264-020-00567-7. Epub 2020 Aug 29. Neurosci Bull. 2020. PMID: 32860626 Free PMC article. Review.
Cited by
-
Molecular hallmarks of ageing in amyotrophic lateral sclerosis.Cell Mol Life Sci. 2024 Mar 2;81(1):111. doi: 10.1007/s00018-024-05164-9. Cell Mol Life Sci. 2024. PMID: 38430277 Free PMC article. Review.
-
Exploring the Causal Relationship Between Telomere Biology and Alzheimer's Disease.Mol Neurobiol. 2023 Aug;60(8):4169-4183. doi: 10.1007/s12035-023-03337-4. Epub 2023 Apr 12. Mol Neurobiol. 2023. PMID: 37046137 Free PMC article. Review.
References
-
- Chuenwisad K., More-Krong P., Tubsaeng P., Chotechuang N., Srisa-Art M., Storer R. J., et al. (2021). Premature Senescence and Telomere Shortening Induced by Oxidative Stress from Oxalate, Calcium Oxalate Monohydrate, and Urine from Patients with Calcium Oxalate Nephrolithiasis. Front. Immunol. 12, 696486. 10.3389/fimmu.2021.696486 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

