Hemicellulosic Polysaccharides From Bamboo Leaves Promoted by Phosphotungstic Acids and Its Attenuation of Oxidative Stress in HepG2 Cells
- PMID: 35769382
- PMCID: PMC9234559
- DOI: 10.3389/fnut.2022.917432
Hemicellulosic Polysaccharides From Bamboo Leaves Promoted by Phosphotungstic Acids and Its Attenuation of Oxidative Stress in HepG2 Cells
Abstract
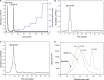
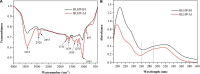
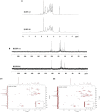
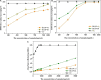
In this work, we exploited an efficient method to release hemicellulosic polysaccharides (BLHP) from bamboo (Phyllostachys pubescens Mazel) leaves assisted by a small amount of phosphotungstic acid. Structural unit analysis proved that BLHP-A1 and BLHP-B1 samples possessed abundant low-branch chains in →4)-β-D-Xylp-(1→ skeleton mainly consisting of Xylp, Manp, Glcp, Galp, and Araf residues. According to the results of the antioxidant activity assays in vitro, both of the two fractions demonstrated the activity for scavenging DPPH⋅ and ABTS+ radicals and exhibited relatively a high reducing ability compared to the recently reported polysaccharides. Moreover, the antioxidant activities of purified polysaccharides were evaluated against H2O2-induced oxidative stress damage in HepG2 cells. BLHP-B1 showed more activity for preventing damages from H2O2 in HepG2 cells by improving the enzyme activities of SOD, CAT, and GSH-Px and decreasing the production of MDA as well as suppressing reactive oxygen species (ROS) formation. This study implied that BLHP could demonstrate its attenuation ability for oxidative stress in HepG2 cells.
Keywords: HepG2 cells; bamboo leaves; hetero-polysaccharides; oxidative stress; phosphotungstic acid.
Copyright © 2022 Xiao, Li, Wang, Zhang, Ge, Mao and Sha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Structural characterization, antioxidant and antimicrobial activity of water-soluble polysaccharides from bamboo (Phyllostachys pubescens Mazel) leaves.Int J Biol Macromol. 2020 Jan 1;142:432-442. doi: 10.1016/j.ijbiomac.2019.09.115. Epub 2019 Oct 5. Int J Biol Macromol. 2020. PMID: 31593720
-
Fractionation and characterization of hemicelluloses from young bamboo (Phyllostachys pubescens Mazel) leaves.Carbohydr Polym. 2013 Jun 5;95(1):262-71. doi: 10.1016/j.carbpol.2013.03.007. Epub 2013 Mar 13. Carbohydr Polym. 2013. PMID: 23618268
-
Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens.Carbohydr Polym. 2020 Feb 1;229:115432. doi: 10.1016/j.carbpol.2019.115432. Epub 2019 Oct 8. Carbohydr Polym. 2020. PMID: 31826528
-
Structural characterization and hepatoprotective activities of polysaccharides from the leaves of Toona sinensis (A. Juss) Roem.Carbohydr Polym. 2019 May 15;212:89-101. doi: 10.1016/j.carbpol.2019.02.031. Epub 2019 Feb 12. Carbohydr Polym. 2019. PMID: 30832884
-
The antioxidant activity of polysaccharides from Armillaria gallica.Front Nutr. 2024 Feb 14;11:1277877. doi: 10.3389/fnut.2024.1277877. eCollection 2024. Front Nutr. 2024. PMID: 38419855 Free PMC article.
Cited by
-
Antioxidant and anti-inflammatory function of Eupatorium adenophora Spreng leaves (EASL) on human intestinal Caco-2 cells treated with tert-butyl hydroperoxide.Sci Rep. 2024 May 7;14(1):10509. doi: 10.1038/s41598-024-61012-7. Sci Rep. 2024. PMID: 38714697 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

